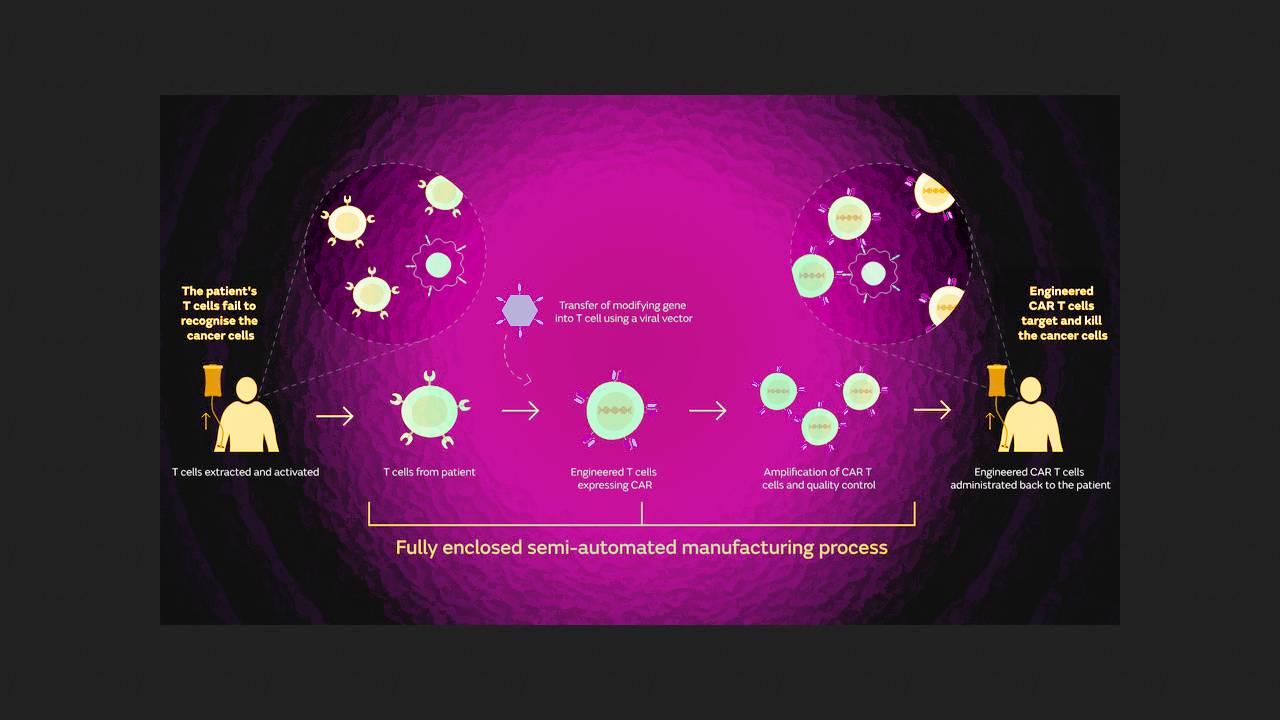
On November 8, 2024, the US Food and Drug Administration (FDA) approved obecabtagen autoleucel for the treatment of relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL). It is an autologous 41BB-ζ anti-CD19 CAR T cell with scFv of intermediate affinity. Results from the FELIX clinical trial, which prompted the FDA's approval, were officially published in the New England Journal of Medicine almost 20 days later. They were conducted by Claire Roddie of University College London and her colleagues from the UK, Spain, and the US.
Of the 94 participants in the Phase 2A multicenter trial, 77 percent achieved remission over a median observation period of 20.3 months, with 55 percent achieving complete remission and 21 percent achieving complete remission with incomplete hematological recovery. Severe (grade 3 or higher) treatment-related adverse events were observed relatively rarely: cytokine release syndrome (CRS) occurred in 2.4 percent of participants, and ICAN syndrome (ICAN) occurred in 7.1 percent of participants.