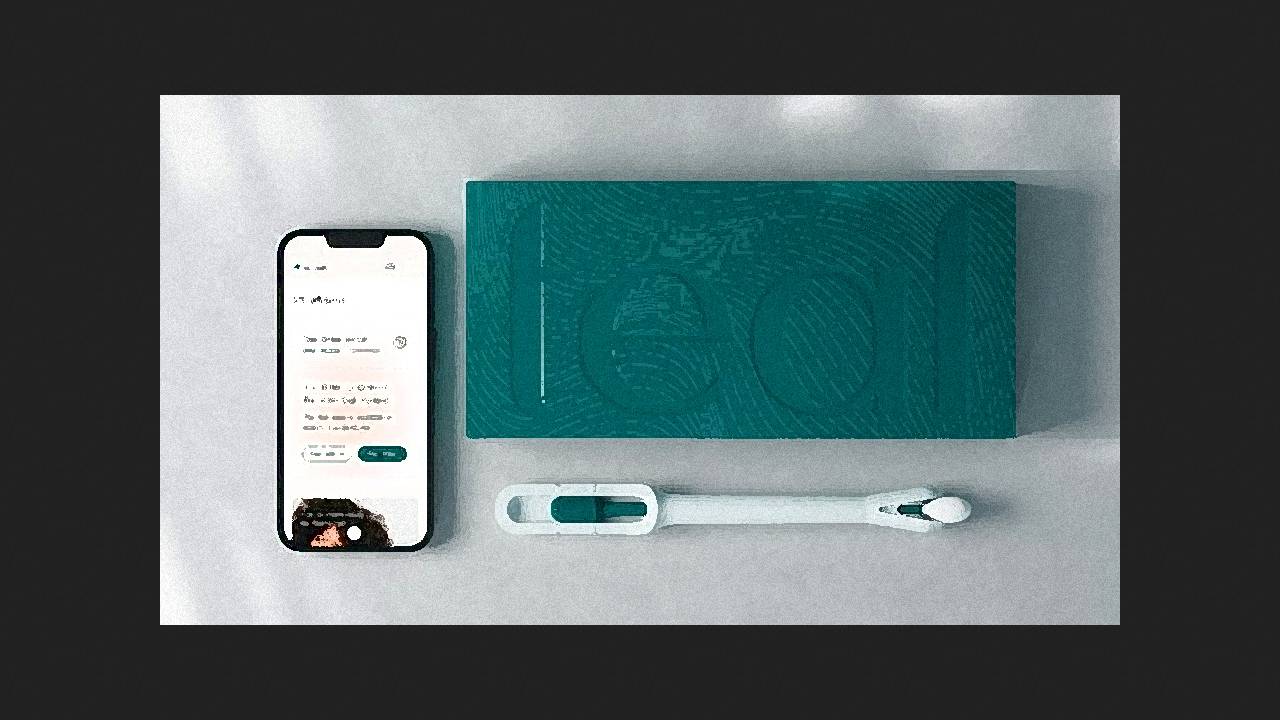
The U.S. Food and Drug Administration (FDA) has approved the first device for self-administered, at-home cervical smear testing for human papillomavirus (HPV) and cancer screening, according to a press release from Teal Health.
Cervical cancer is a common malignancy (including among young women), most often caused by certain types of the human papillomavirus (HPV). It is one of the few cancers that is almost completely preventable through screening. For the past 80 years, the Pap smear has been the standard screening method. It is performed by a physician using a speculum and a cervical brush; the resulting smear is stained and examined under a microscope. Recently, microscopy has been replaced by more sensitive primary HPV screening using a Pap smear.
Despite the high effectiveness of screening, its coverage is declining, especially in developed countries. This is due to difficulties in scheduling and finding appointments with a doctor, as well as the discomfort associated with the procedure. In 2024, the FDA approved two devices from BD and Roche aimed at addressing the latter issue—they allow women to perform a swab test themselves, but in a healthcare setting.
The Teal Wand kit is designed for self-collection of cervical smears at home by women aged 25 to 65 years with an average risk of cervical cancer. It includes a sponge swab with a guide for collecting the smear (the manufacturer claims it's no more difficult to use than a regular sanitary swab) and a container for sending the sample to an affiliated laboratory, where it is analyzed using the Roche cobas HPV clinical diagnostic system. The kit is integrated with a telemedicine service, providing access to a Teal physician, who prescribes the kit (available by prescription only), guides the woman through the smear collection process, interprets the laboratory report, and advises on next steps.
The FDA's decision was prompted by the results of the SELF-CERV clinical trial, conducted with over 600 women at 16 U.S. sites. These trials confirmed that Pap smears taken with the Teal Wand are as effective as those taken by a professional in terms of diagnostic efficacy, detecting precancerous cervical changes in 96 percent of cases. Furthermore, 94 percent of participants reported they would prefer to take a Pap smear at home if they were confident in its effectiveness, and 86 percent said they would be much more likely to undergo screening on time if they were tested this way.
Teal Wand kits are expected to be available in California as early as June 2025, with rollout to other U.S. states soon after. The estimated price for the service has not yet been announced, but Teal is working with all major health insurance companies to make it as accessible as possible.
Previously, the FDA licensed over-the-counter tests for the rapid self-diagnosis of chlamydia, gonorrhea, and trichomoniasis using a vaginal swab in women, and syphilis using a drop of blood from a finger prick in men and women.