Chemists from Spain and the United States have developed a new method for synthesizing macrocycles. They obtained them from covalent organic frameworks containing double bonds in their structure. The chemists cleaved these bonds using ozonolysis, producing rings of over a hundred atoms. Macrocycles of this type had previously been impossible to obtain, the study's authors write in the journal Science.
Macrocyclic molecules are typically prepared through macrocyclization reactions, which involve the closure of a linear molecule into a ring. These reactions often have low yields because the molecules react more quickly with each other than with themselves. As a result, the reaction mixture must be highly diluted to reduce the number of molecules encountering each other, which slows the reaction rate.
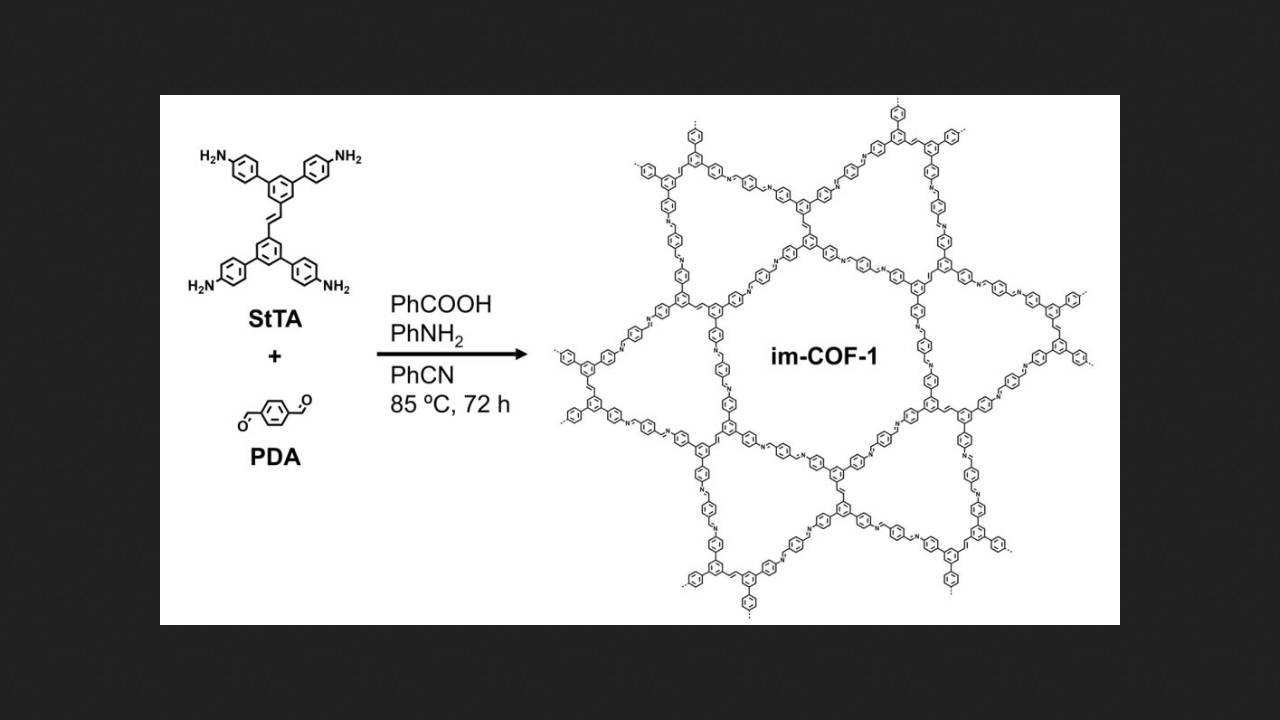
A method for synthesizing macrocycles without macrocyclization was proposed by chemists led by Jorge Albalad of the Autonomous University of Barcelona. Their idea was to first obtain covalent organic frameworks containing double bonds and then cleave these bonds to release the desired macrocycles from the framework.
The researchers began by synthesizing a covalent organic framework from substituted stilbene with four amino groups and terephthalaldehyde. The two-dimensional structure of this framework was a kagome-type structure, or trihexagonal mosaic, in which hexagonal and triangular pores alternate. Moreover, the double bonds were located in the triangular pores, so rupturing them would not damage the hexagonal pores.
Next, the chemists oxidized all the imine groups in the framework to amide groups to prevent the imines from being destroyed during ozonolysis, and then performed the ozonolysis itself. The scientists bubbled ozone through a yellow suspension of the framework in a mixture of N,N-dimethylformamide, tetrahydrofuran, and methanol at -78 degrees Celsius for 10 minutes. After the reaction was complete, they obtained a colorless, transparent solution, to which they added dimethyl sulfide. As a result, all the carbon-carbon double bonds in the framework were destroyed, and in their place, each carbon atom formed a double bond with oxygen.
As the chemists predicted, the reaction product was a macrocycle, which they purified using size-exclusion chromatography and characterized using NMR spectroscopy and mass spectrometry. A similar framework, but with carboxyl groups instead of aldehyde ones, was obtained by using oxone instead of dimethyl sulfide in the second step of the ozonolysis reaction. Both resulting macrocycles contained 114 ring atoms.
Thus, the chemists proposed a new approach to the synthesis of polyamide macrocycles. They succeeded in obtaining several more products with 138 and 162 ring atoms, as well as a polyimide macrocycle with 114 ring atoms—all in near-quantitative yields using gram-scale starting materials.
Covalent organic frameworks are often used for gas absorption. For example, we recently reported on how chemists synthesized the first covalent organic framework capable of selectively absorbing up to two millimoles of carbon dioxide per gram of absorbent within an hour.