Physicists observed how water with drops of silicone oil freezes and discovered that at a high freezing front speed (about 1.6 micrometers per second), a drop of oil deforms the ice, as if pressing into it instead of being pushed out. Scientists explained this seemingly paradoxical interaction of a foreign inclusion and a solidifying liquid by the thermal Marangoni effect. The results of the study were published in the journal Physical Review Letters.
When water containing impurities and inclusions (such as air bubbles or sand) freezes, the foreign particles usually either become part of the ice or are forced out by the freezing front. The conditions on which the absorption or expulsion of the impurity depends depend on the interaction between the particle itself and the moving solidification wave. At the molecular level, this interaction is controlled by the Van der Waals forces and the flow of liquid in a thin film around the particle. At the same time, at the macro level, the main role is played by heat exchange between the inclusion and the surrounding substance, while the dispersed particle itself experiences deformation due to the difference in thermal conductivity between the foreign object and the liquid around it. By studying such a physical model, scientists have discovered another remarkable fact - for a solid inclusion, the deformation does not depend on how quickly the freezing front approaches, and the deformation of the resulting ice is directed toward the particle.
Pallav Kant from the University of Manchester, together with colleagues from the UK, Germany and the Netherlands, discovered the paradoxical behaviour of a drop of oil placed in freezing water: instead of being displaced or absorbed by the liquid, it deformed the solidifying liquid.
To conduct the experiment, the scientists used two transparent plates, located parallel to each other at a distance of 200 micrometers, the space between which was filled with an emulsion of silicone oil in water, thus forming Hele-Shaw flows in the setup. To stabilize the mixture and avoid unexpected mixing of oil with water, the experimenters added a surfactant, the share of which was one hundredth of a percent of the volume of the final mixture. The scientists created a temperature gradient by moving a cell with water and oil through a refrigeration unit, in which a gradual change in temperature was provided by several Peltier elements.
It turned out that at a low speed of movement (about 0.4 micrometers per second) of the Hele-Shaw cell through the refrigeration unit (and, accordingly, a slow formation of the freezing front), the initially flat solidification boundary was deformed towards the drop, as if attracted to it, which completely coincided with theoretical predictions: the thermal conductivity of a drop of silicone oil is lower than that of water, so the heat flow chose the path of least thermal resistance, avoiding the impurity itself and rejecting the isotherms, which are easily found from the heat conductivity equation for small Peclet numbers.
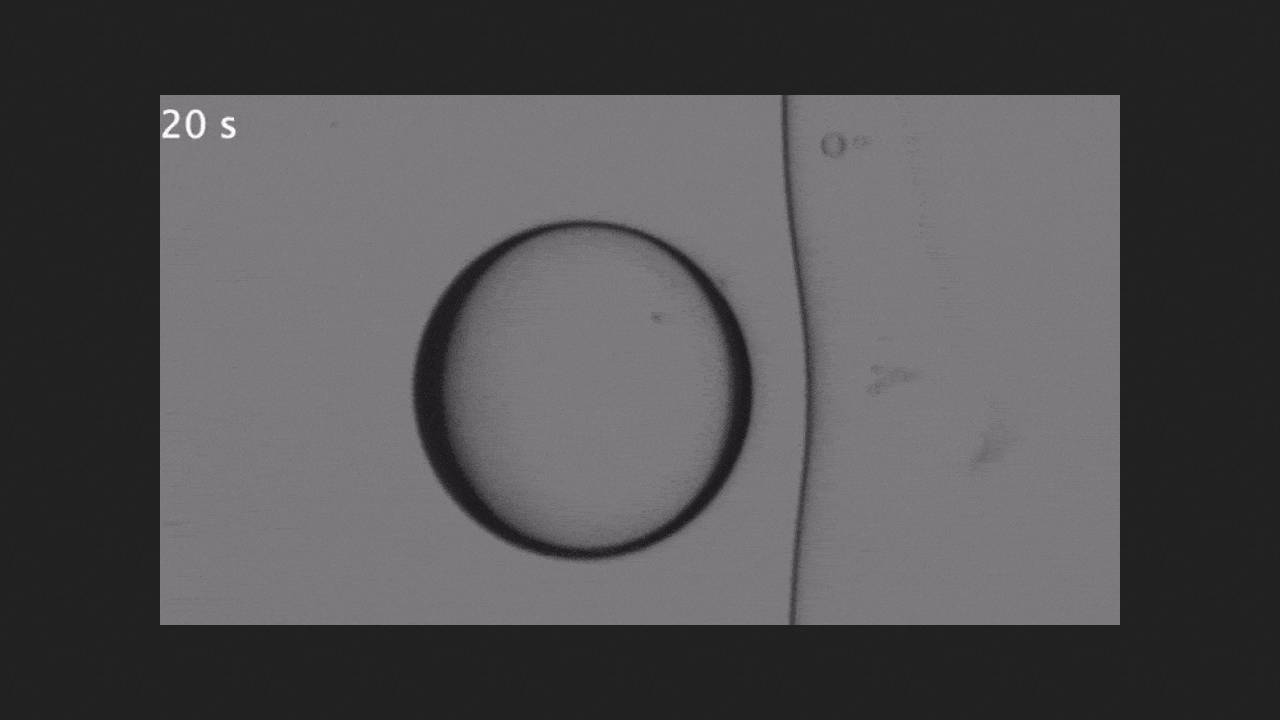
At a freezing front speed of 0.9 micrometers per second, the latter remained flat as it approached the droplet, and at a speed of 1.6 micrometers per second, the droplet was pressed into the solid ice that had formed. Physicists explained this unexpected result as follows: with a rapid decrease in temperature, the surface tension coefficient of silicone oil changes very sharply, so the front of the droplet experiences a greater surface tension force, which in turn causes an interphase flow that carries the liquid from warmer areas to colder ones - the so-called Marangoni heat flow.
The authors of the work noted that the obtained results should be useful in materials science for monitoring processes that determine the rejection or capture of particles during solidification of a multiphase medium.
The interaction of water ice and foreign particles has become the subject of research more than once. We have previously written about how external impurities reduced the adhesion of ice to surfaces.