Swiss chemists discovered that urea readily forms from ammonia and carbon dioxide when the reaction is carried out in microdroplets of water. They used Raman spectroscopy to detect urea formation in individual microdroplets and then reproduced the experiment in an aerosol. The results of the study were published in the journal Science.
The urea molecule consists of two amino groups attached to a carbonyl group, and is essentially a double amide of carbonic acid. Consequently, it can be produced from gaseous ammonia NH3 and carbon dioxide CO2. The problem is that this reaction only occurs under harsh conditions. For example, industrial urea synthesis is carried out at a temperature of approximately 200 degrees Celsius and a pressure of approximately 15 atmospheres. Under normal conditions, ammonia and carbon dioxide simply do not react. However, bubbling a mixture of these gases through water produces ammonium carbamate, not urea.
But, as it turns out, urea can indeed form from carbon dioxide and water at atmospheric pressure and room temperature. This was demonstrated by Ruth Signorell and her colleagues at the ETH Zurich. The scientists conducted this reaction not in ordinary water, but in water droplets with a radius of several micrometers.
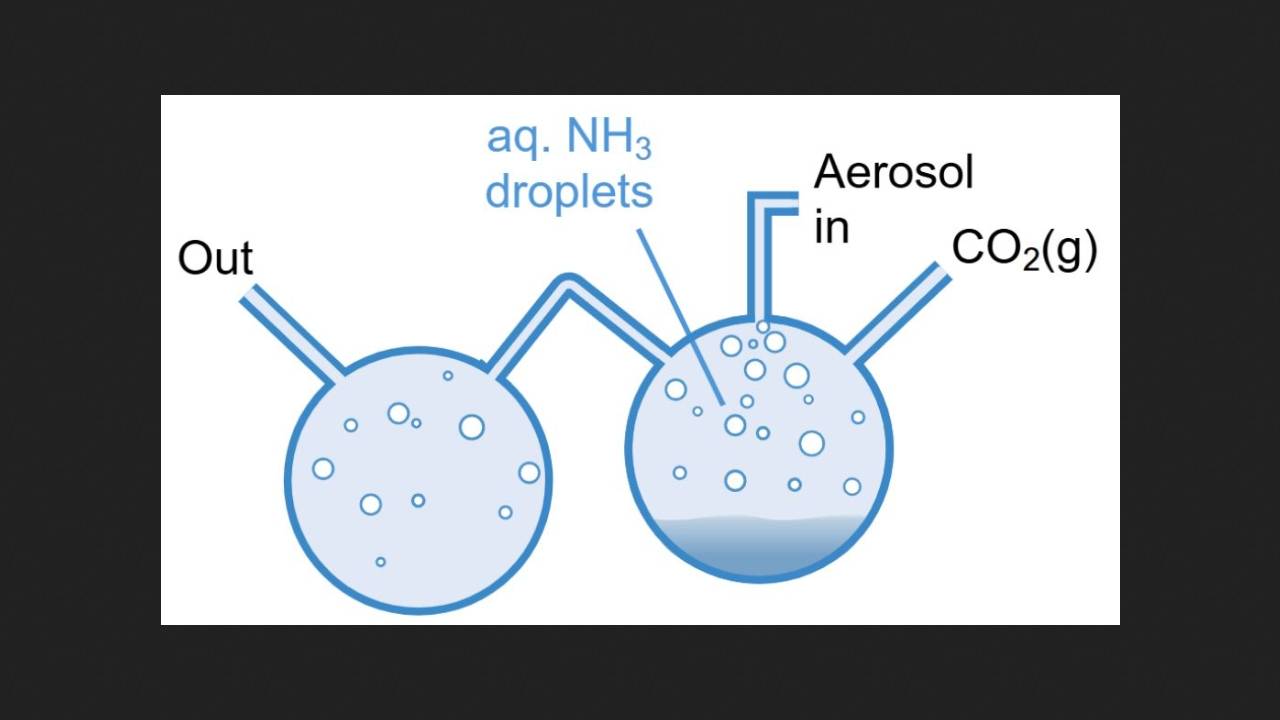
The chemists conducted the experiment by spraying an aqueous ammonia solution and capturing the individual microdroplets that formed using optical tweezers. Next, they introduced one atmosphere of humid carbon dioxide into the chamber containing the droplet, simultaneously recording its Raman spectrum. A few minutes after the reaction began, a band corresponding to the vibration of the nitrogen-carbon bond in urea appeared in the spectrum. The urea concentration in the droplet after 30 minutes of reaction averaged approximately 40 millimoles per liter. The temperature during the experiment was 20 degrees Celsius.
The chemists then conducted the same reaction, but not in a single drop of water, but in an aerosol. This also produced urea, which the scientists detected using mass spectrometry and carbon NMR spectroscopy. However, in this case, the amount of urea produced was much smaller because the lifetime of the microdroplets, and therefore the reaction time, was about three minutes.
The study's authors then repeated the experiment in regular water, just to be on the safe side. In this case, as they expected, no urea was produced. The role of microdroplets is presumably due to the difference in concentrations of substances within the droplet and on its surface. Each droplet represents a small flow reactor, with different conditions in different areas, including varying pH levels. This concentration gradient, the chemists believe, is what allows urea to form. However, they also note that the reaction mechanisms in microdroplets are very difficult to determine and often become the subject of debate in the scientific community.
Thus, chemists demonstrated that urea can be formed from ammonia and carbon dioxide under normal conditions. This is precisely how, the scientists suggest, urea could have formed under prebiotic conditions.
Previously, we reported on how ammonia entered microdroplets of water from the air exhaled by experimenters.