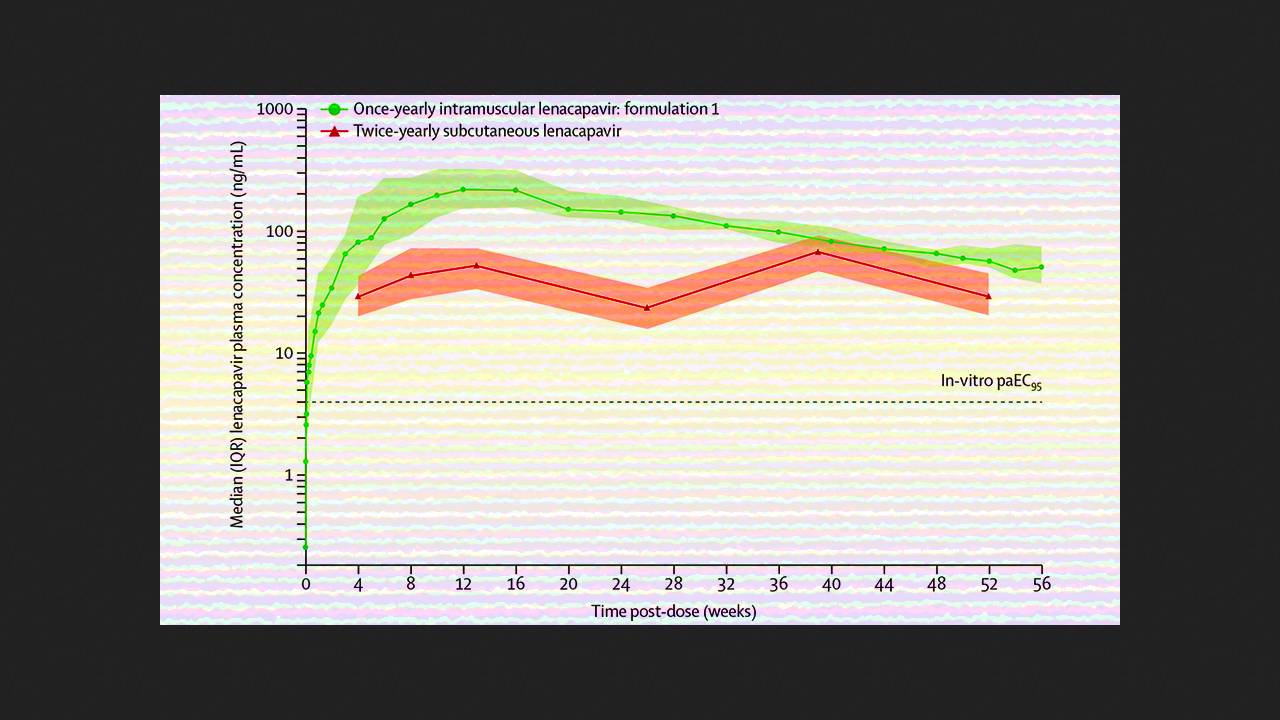
Vamshi Jogiraju and colleagues at Gilead Sciences reported the success of a phase I clinical trial of lenacapavir as a single-year injection for HIV pre-exposure prophylaxis. According to a publication in The Lancet, the new formulation of this viral capsid inhibitor provided even more stable plasma concentrations than twice-yearly administration. It consists of lenacapavir free acid in 5% or 10% ethanol and is administered at a single dose of 5,000 milligrams via two intramuscular ventrogluteal injections. The previous injectable formulation is administered subcutaneously every six months.
In a trial involving 32 adults, one year after a single intramuscular injection, the median plasma concentration of the drug was 57.0–65.6 nanograms per milliliter, compared to 23.4 nanograms per milliliter after six months with the previous formulation administered subcutaneously. The most common side effect was pain at the injection site, which resolved within a week. Twice-yearly lenacapavir injections have already demonstrated effective pre-exposure prophylaxis for HIV infection in Phase III clinical trials and have been named a major breakthrough of 2024 by the journal Science.