British chemists discovered that iron complexes with N-heterocyclic carbenes can catalyze Suzuki cross-coupling. The reactants were aromatic boronic acid esters and aryl chlorides, and the reaction resulted in the formation of substituted biaryls. The study was published in the journal Nature Synthesis.
The Suzuki reaction creates a carbon-carbon bond between two organic molecules—a boronic acid and a halide. The cross-coupling is catalyzed by various palladium(0) complexes with phosphines, N-heterocyclic carbenes, and other ligands. In 2010, Japanese chemist Akira Suzuki received the Nobel Prize for his discovery of this reaction.
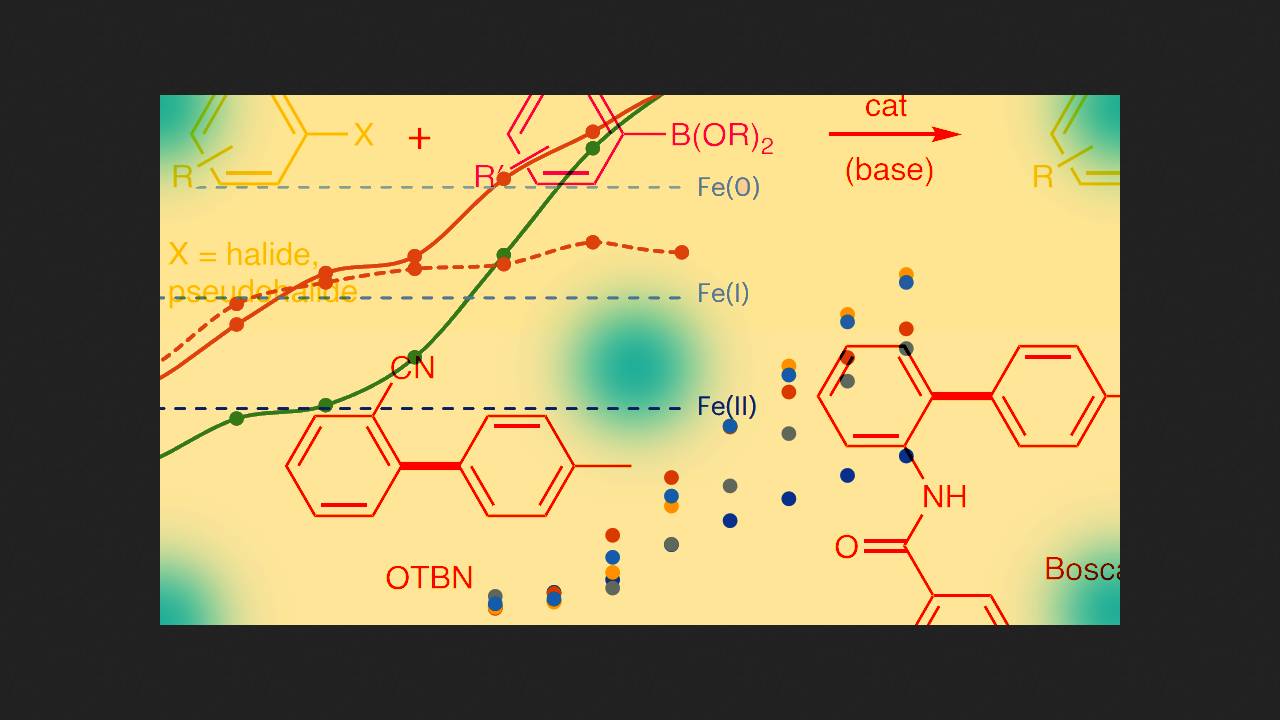
Several years ago, chemists led by Robin B. Bedford at the University of Bristol decided to investigate whether the Suzuki reaction could be performed with cheaper iron-based catalysts. In 2018, they discovered that it was possible: in the presence of an iron carbene complex, aryl halides reacted with boronic acid esters to form a cross-coupling product. However, only aryl halides containing a directing group adjacent to the halogen reacted.
Recently, the same scientists discovered that if the reaction conditions are carefully chosen, the directing group is unnecessary. The chemists conducted the reaction as follows: they mixed an aryl chloride, a boronic acid ester, magnesium bromide, methyl magnesium bromide, iron (+3) bromide, and a source of carbene ligand in a mixture of dioxane and 2-methyltetrahydrofuran. The scientists then heated the mixture for several hours at 100 degrees Celsius, yielding the cross-coupling product. As the chemists note, in most experiments, a homocoupling product of boronic ester molecules was formed as a byproduct, which had to be separated by chromatography.
The scientists further demonstrated that their reaction worked with a variety of boron derivatives and aryl chlorides, with conversion yields in some cases reaching 90 percent. However, if one of the starting materials contained a carbonyl group or an unsubstituted amino group, the desired product was not formed.
Thus, chemists have discovered a new method for performing the Suzuki reaction using an iron catalyst. The researchers haven't yet determined which complex acts as the catalytically active species. However, they hypothesize that in the first stage of the reaction, iron is reduced to the +1 oxidation state and then oxidatively adds the aryl chloride.
Previously, we talked about how chemists learned to produce diarylamines using a modification of the Suzuki reaction.