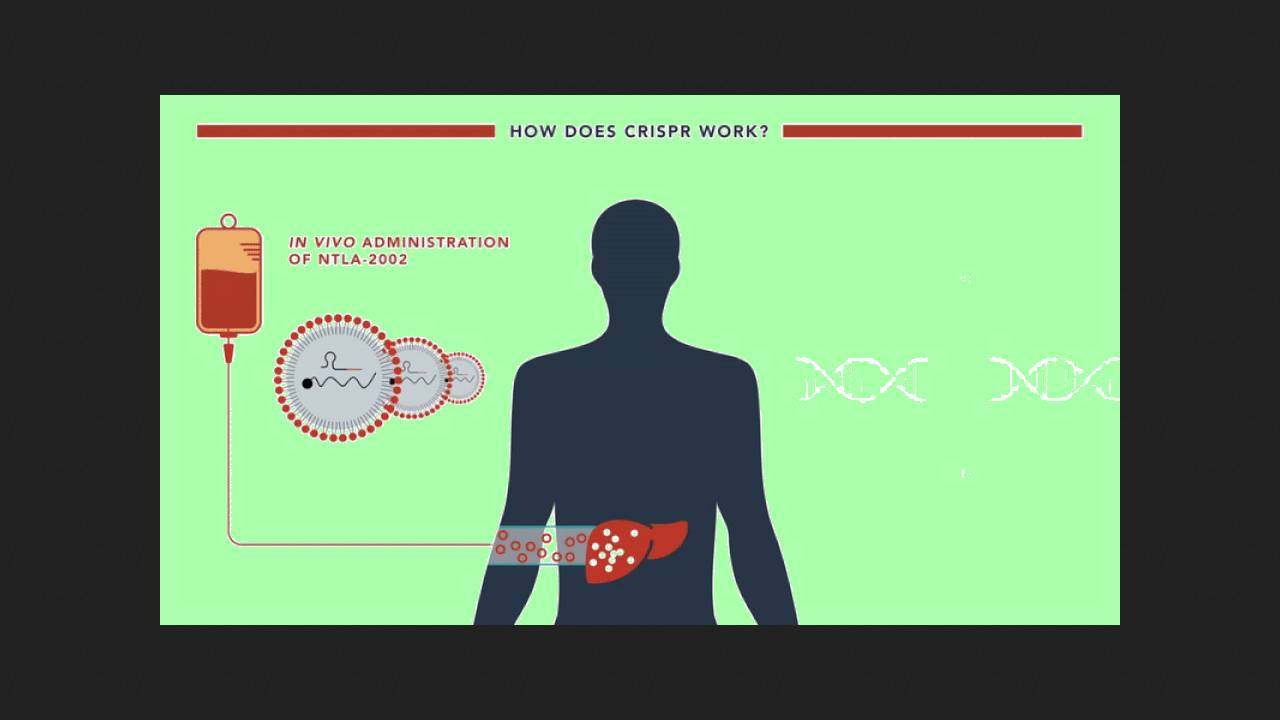
Danny Cohn of the University of Amsterdam and colleagues from six countries reported the success of a phase II in vivo clinical trial of CRISPR therapy for hereditary angioedema. The experimental drug, NTLA-2002, contains components of the CRISPR/Cas9 system packaged in lipid nanoparticles. When administered, these components silence the KLKB1 gene, which is associated with the disease (we previously wrote in detail about its mechanism of action and trial design). A single dose should provide lifelong benefits. In the phase II trial, 10 patients received 25 milligrams of the drug, 11 patients received 50 milligrams, and six received a placebo. The results were published in The New England Journal of Medicine.
Compared with placebo, during the first 16 weeks, the frequency of attacks was reduced by an average of 75 percent at the 25-milligram dose and by 77 percent at the 50-milligram dose; plasma kallikrein levels were reduced by 55 and 86 percent compared to baseline. Furthermore, after administration of these doses of the active drug, 40 percent and 73 percent of patients, respectively, remained attack-free during the observation period without any additional therapy. The most common side effects of NTLA-2002 included headache, increased fatigue, and nasopharyngitis.