Researchers from six countries presented the results of a pilot clinical trial of a system consisting of an ocular implant and glasses with a processor for vision restoration in patients with geographic retinal atrophy. Eighty percent of patients experienced a clinically significant improvement in visual acuity. A report of the study was published in The New England Journal of Medicine.
Age-related macular degeneration (AMD) is a chronic disease that affects the photoreceptors of the macula (the area of the retina responsible for central, most acute color vision). It is the leading cause of blindness in people aged 60 and older. Geographic atrophy is a late stage of dry AMD, characterized by progressive, irreversible loss of photoreceptor cells and retinal pigment epithelium, leading to vision loss. There are currently no effective treatments for this disease.
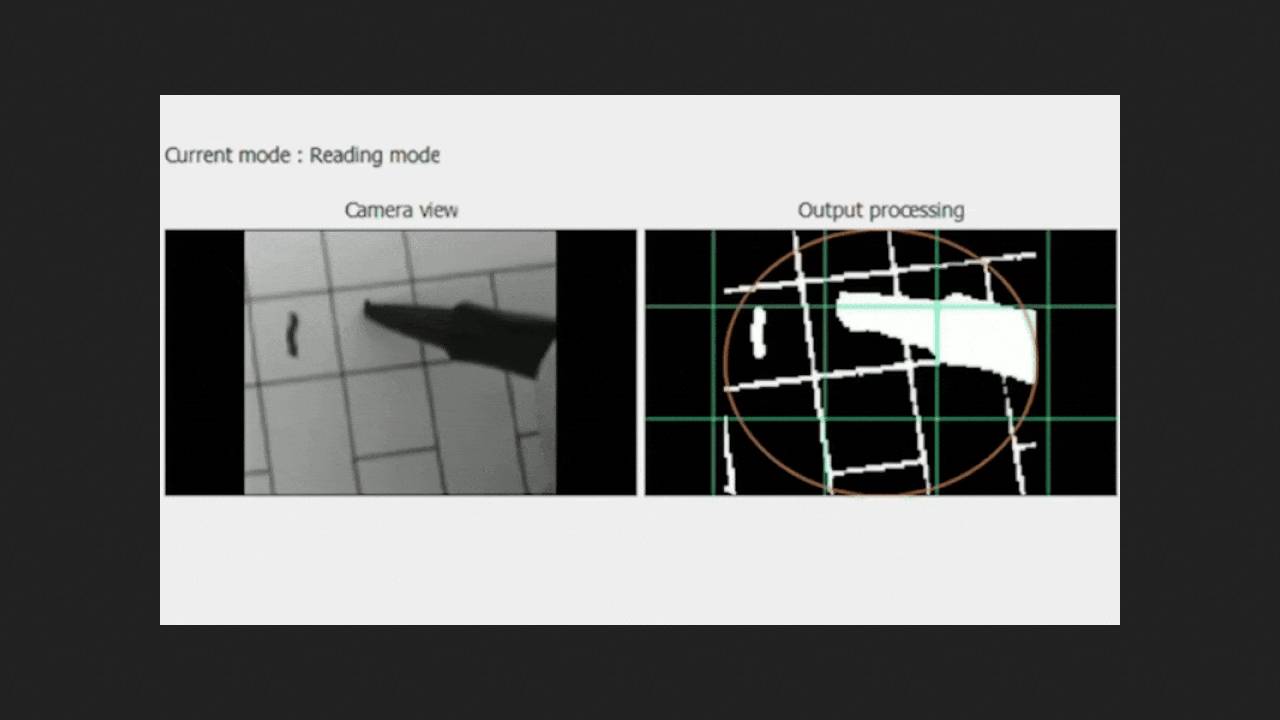
To help such patients, the PRIMA (photovoltaic retina implant microarray) system was developed ten years ago. It is based on a crystalline silicon matrix measuring two by two millimeters and 30 micrometers thick with 378 pixels each 100 micrometers wide, which is implanted under the atrophied area of the retina. A camera attached to prescription glasses captures visible light and sends the image to a pocket-sized processor, which converts it into near-infrared radiation with a wavelength of 880 nanometers, with adjustable magnification and brightness. This image is projected onto the implanted matrix, which converts it into electrical impulses that excite the bipolar cells of the retina, which transmit information along the optic nerve to the brain. Central vision from the implant is combined with natural peripheral vision.
Now, Frank Holz of the University of Bonn, along with colleagues from the UK, Germany, Italy, the Netherlands, the US, and France, and Science Corporation, have conducted open-label, non-randomized prospective trials of PRIMAvera at 17 European clinical centers. Thirty-eight patients aged 60 years or older (mean 78.9 years) with AMD-related geographic retinal atrophy in both eyes, confirmed by fundus autofluorescence and optical coherence tomography, visual acuity of at least 1.2 logarithms of the minimum angle of resolution (LogMAR), and a macular atrophy diameter greater than 2.4 millimeters (i.e., larger than the implant) participated in the trial. All patients received the PRIMA implant in one eye and were trained in the use of the system.
After 12 months, three participants had died, one refused to participate, two did not contact the investigators, and the remaining 32 were available for evaluation of the effect. Of these, 30 perceived signals from the implant in the central visual field, and 26 (81 percent; p < 0.001) experienced a clinically significant improvement in visual acuity (at least 0.2 logMAR, average 0.49 logMAR, maximum 1.18 logMAR). Moreover, a minimally significant improvement was observed in 20 of 35 participants after six months. At 12 months, 27 of 32 (84 percent) participants could read letters, numbers, and words with the device; 69 percent reported moderate or high satisfaction with the treatment. A total of 26 serious adverse events were recorded in 19 participants during the follow-up period. 21 of these occurred within two months after surgery, and 20 resolved within the next two months. Natural peripheral visual acuity remained unchanged from baseline. Pilot trials indicate that the PRIMA system can restore the ability to perform everyday tasks such as reading and writing in most patients with geographic retinal atrophy and significant vision loss. Further experiments may further enhance this effect by implanting multiple microarrays or a higher-resolution microarray. Previously, American scientists successfully halted the progression of AMD in four patients by implanting retinal pigment epithelium derived from stem cells. Melatonin or turmeric supplementation have also been shown to reduce the risk of AMD.