German chemists have discovered how to cross-couple anilines with boronic acids. The anilines were converted into diazonium salts, which then oxidatively added to a palladium catalyst. The study was published in Angewandte Chemie International Edition.
In the classic Suzuki cross-coupling, a boronic acid and an aryl halide react to form a carbon-carbon bond in the presence of a palladium catalyst. Anilines can be used in place of aryl halides in this reaction, but this requires first converting them to unstable and sometimes explosive diazonium salts.
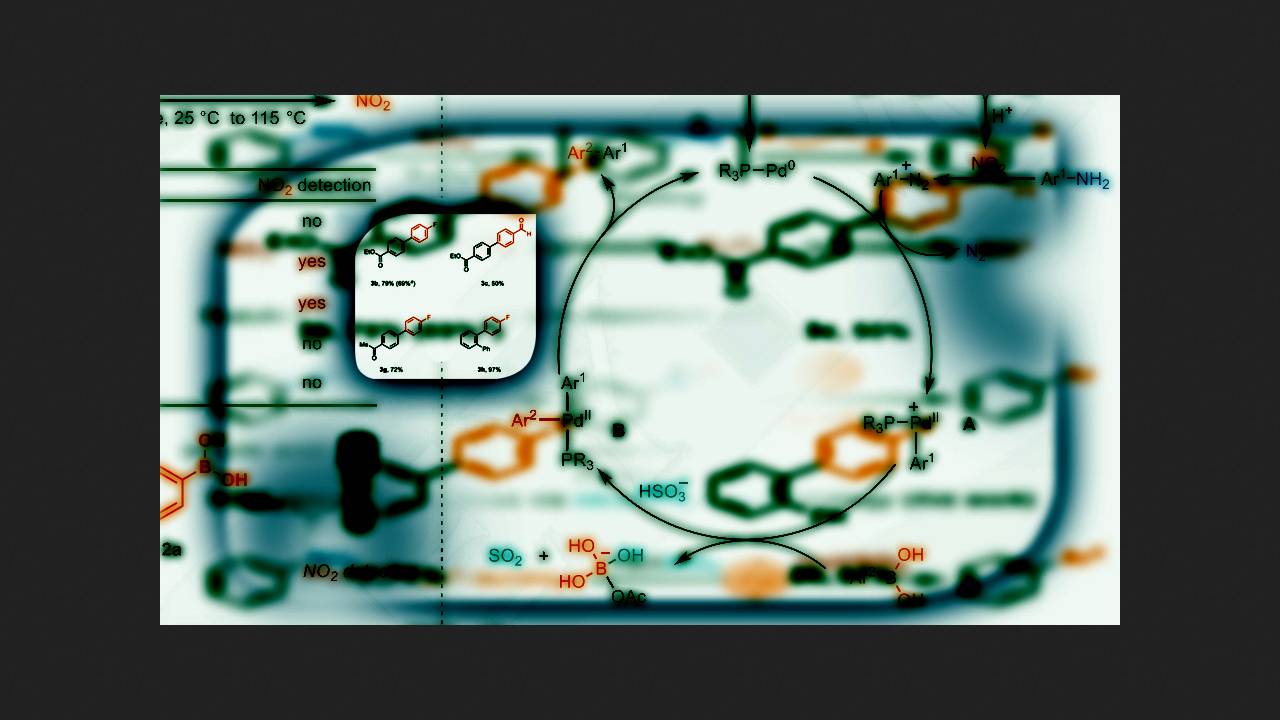
To overcome this problem, chemists led by Tobias Ritter from the Max Planck Institute for Coal Research decided to apply a recently developed method for generating diazonium salts from anilines using nitrates. They had previously used this method to produce aromatic halogen derivatives from anilines, and it proved safer than classical diazotization because diazonium salts did not accumulate in the reaction mixture.
For the same reason, the chemists decided to apply this method to the Suzuki reaction with anilines. They first mixed 4-fluoroboronic acid and 4-methylaniline in the presence of various palladium catalysts, tert-butylammonium nitrate, and other additives. They found that the reaction proceeded with a yield of 69 percent when sodium hydrosulfite (NaHSO3) was used as the reducing agent. In their previous work, the scientists used sodium thiosulfate (Na2S2O3), but they suspected that it bonded too strongly with the palladium catalyst, and the Suzuki reaction practically did not proceed in its presence.
The chemists tested the selected reaction conditions on various anilines and boronic acids. It turned out that these conditions allowed the use of not only anilines but also amino heterocycles. Furthermore, due to the rapid oxidative addition of diazo compounds, the reaction was selective even in the presence of aromatic bromine derivatives. The main drawback of this reaction, as the authors note, is the low yields of products when using boronic acids with donor substituents on the aromatic ring.
Thus, the chemists developed a general method for synthesizing biaryl derivatives from anilines and boronic acids. They used a safer nitrate method to generate diazo compounds, and most products were obtained in yields of 40 percent or more.
We recently reported on how scientists used an iron-based catalyst, rather than palladium, to carry out the Suzuki reaction.