Physicists observed the freezing of water containing droplets of silicone oil and discovered that at high freezing front speeds (approximately 1.6 micrometers per second), the oil droplet deforms the ice, appearing to be pressed into it instead of being pushed outward. The scientists explained this seemingly paradoxical interaction between a foreign inclusion and a solidifying liquid using the thermal Marangoni effect. The results of the study were published in the journal Physical Review Letters.
When water containing impurities and inclusions (such as air bubbles or sand) freezes, the foreign particles typically either become part of the ice or are expelled by the freezing front. The conditions that determine whether an impurity is absorbed or expelled depend on the interaction between the particle itself and the moving freezing wave. At the molecular level, this interaction is controlled by van der Waals forces and the flow of liquid in a thin film around the particle. Meanwhile, at the macroscopic level, heat exchange between the inclusion and the surrounding substance plays a key role, with the dispersed particle itself experiencing deformation due to the difference in thermal conductivity between the foreign object and the surrounding liquid. By studying this physical model, scientists discovered another remarkable fact: for a solid inclusion, deformation is independent of the speed of the freezing front approach, and the deformation of the forming ice is directed toward the particle.
Pallav Kant of the University of Manchester, together with colleagues from the UK, Germany and the Netherlands, discovered the paradoxical behaviour of a drop of oil placed in freezing water: instead of being displaced or absorbed by the liquid, it deformed the solidifying liquid.
To conduct the experiment, the scientists used two transparent plates placed parallel to each other at a distance of 200 micrometers. The space between them was filled with an emulsion of silicone oil in water, thus forming Hele-Shaw flows in the setup. To stabilize the mixture and prevent unintended mixing of the oil and water, the experimenters added a surfactant, whose proportion was one hundredth of a percent of the final mixture's volume. The scientists created a temperature gradient by moving a cell containing water and oil through a refrigeration system, in which a gradual temperature change was achieved using several Peltier elements.
It turned out that at a low speed of movement (about 0.4 micrometers per second) of the Hele-Shaw cell through the refrigeration unit (and, accordingly, the slow formation of the freezing front), the initially flat solidification boundary was deformed towards the droplet, as if attracted to it, which completely coincided with theoretical predictions: the thermal conductivity of a droplet of silicone oil is lower than that of water, so the heat flow chose the path of least thermal resistance, avoiding the impurity itself and rejecting isotherms that are easily found from the heat conductivity equation for small Peclet numbers.
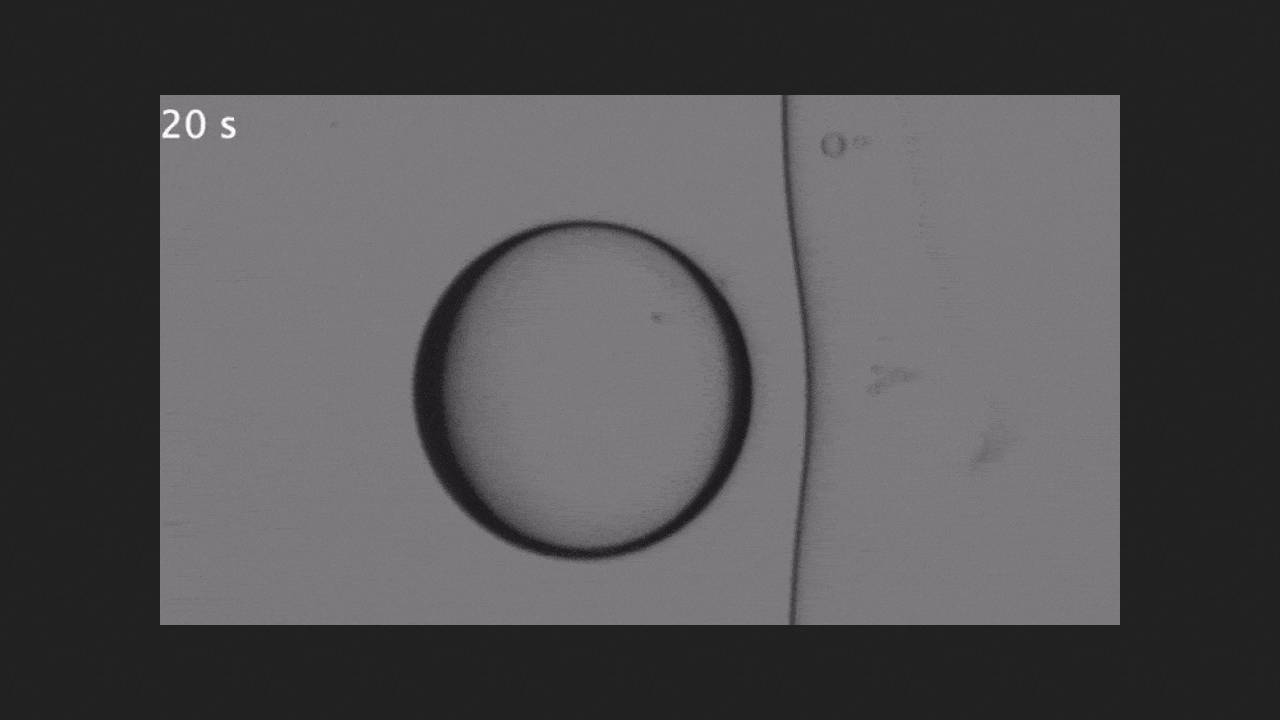
At a freezing front velocity of 0.9 micrometers per second, the latter remained flat as it approached the droplet, while at a velocity of 1.6 micrometers per second, the droplet was pressed into the formed solid ice. Physicists explained this unexpected result as follows: with a rapid decrease in temperature, the surface tension coefficient of silicone oil changes very sharply, causing the front of the droplet to experience a greater surface tension force, which in turn causes interfacial flow, which draws liquid from warmer areas to colder ones—the so-called Marangoni heat flux.
The authors of the work noted that the obtained results should be useful in materials science for monitoring the processes that determine the rejection or capture of particles during the solidification of a multiphase medium.
The interaction of water ice and foreign particles has been the subject of research before. We previously wrote about how external impurities reduced ice adhesion to surfaces.