Physicists cooled TmVO4 from 5 to 2.36 Kelvin using the elastocaloric effect—a phenomenon in which stretching or compressing a material causes its temperature to decrease. The scientists also observed the nonlinear nature of the process: elastocaloric cooling reached its maximum at an initial sample temperature of 3.6 Kelvin. The results of the study were published in Physical Review Applied.
To achieve temperatures of a few kelvins or lower for macroscopic amounts of matter, physicists typically use one of two proven methods: liquid helium cooling or adiabatic nuclear demagnetization. The former requires an expensive isotope of helium, which is extremely rare in nature, while the latter requires bulky and complex equipment generating strong magnetic fields, leading scientists to constantly search for new ways to achieve ultra-low temperatures.
The elastocaloric effect is one of the alternative candidates for cryogenic technologies. This effect essentially states that when adiabatically (i.e., without heat exchange with the environment) stretched or compressed, a material loses some of its internal energy, thereby cooling. However, two problems currently exist: first, researchers have not yet developed methods for measuring the elastocaloric effect at subzero temperatures, and second, few materials with suitable properties have been discovered.
Mark Zic of Stanford University, together with colleagues from the US, demonstrated cooling in the ultra-low temperature region using thulium vanadate, which exhibits elastocaloric properties due to the Jahn-Teller effect: under asymmetric deformation, the ground-state doublet of the Tm3+ ion splits, absorbing energy.
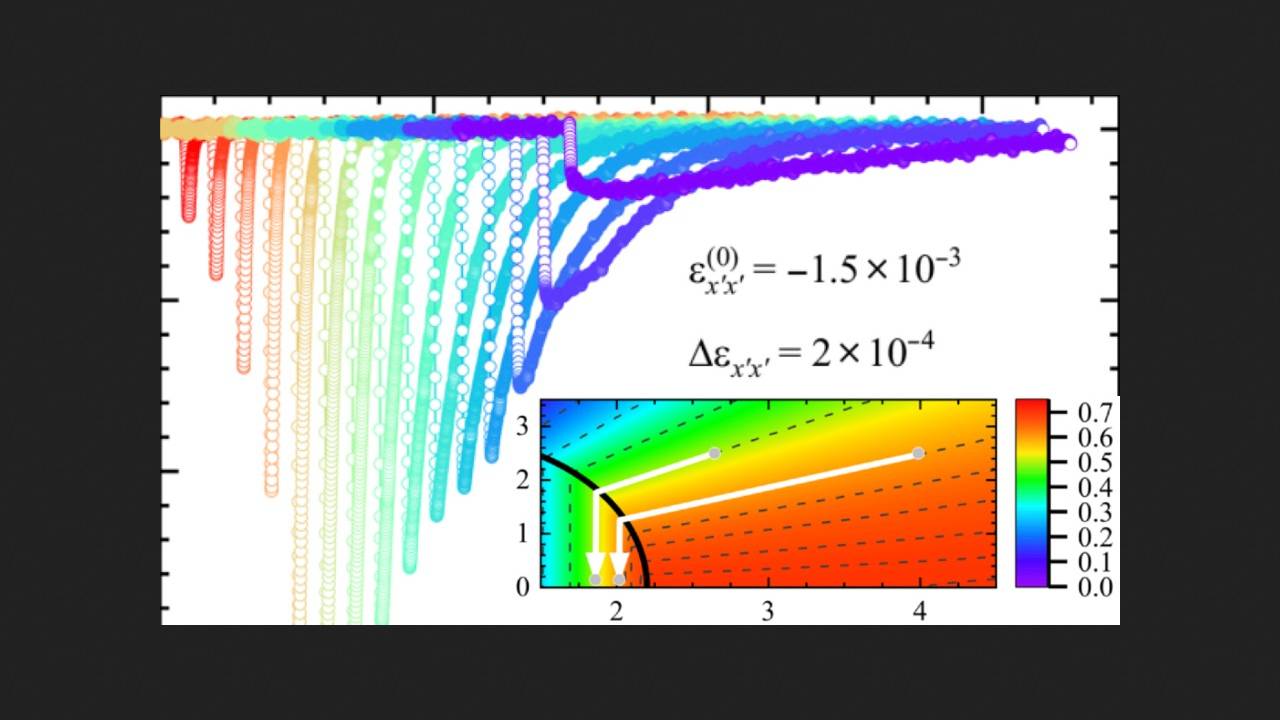
To do this, the physicists grew single crystals of TmVO4 and placed them between the plates of a strain gauge. When a voltage was applied, the strain gauge deformed the samples in two different directions, creating an asymmetric curvature of the crystal lattice. Before each new experiment, the scientists first measured the initial temperature of the material using a ruthenium dioxide sensor and set the initial strain (in most experiments, this was a compression of -2.7 × 10-3). They then applied a pulse of approximately one second to the strain gauge, which deformed the sample by stretching or compressing it.
As a result, the physicists observed that as the strain increased, so did the cooling of the sample. However, upon reaching a value of -1.8 × 10-3 (the minus sign denotes compressive strain), the cooling of the material significantly decreased. Moreover, the elastocaloric effect was virtually absent at an initial temperature of 8 Kelvin for thulium vanadate and reached its maximum at 3.6 Kelvin. The maximum experimental cooling recorded by the scientists was approximately 2.64 Kelvin (the sample cooled from 5 to 2.36 Kelvin) at a tensile strain of approximately 1.8 × 10-3. To evaluate the efficiency of the developed cooler, the researchers calculated its volumetric specific power, which was 0.34 watts per cubic centimeter for a sample weighing 0.19 milligrams.
Since the elastocaloric properties of the material arise due to the Jahn-Teller effect, the authors of the study also proposed using nuclear magnetic resonance and Raman scattering to search for new candidates and test their cryogenic capabilities.
We previously wrote about how physicists used another exotic method—"dark" states of particles—to cool a cloud of molecules to a record-low temperature.