Australian, American, British, and Dutch researchers reported success in phase II clinical trials of a small interfering RNA-based drug for treating elevated lipoprotein (a) levels. The study was published in the Journal of the American Medical Association.
Lipoprotein (a), or Lp(a), is a plasma lipoprotein resembling low-density lipoproteins (LDL, the reservoir of "bad" cholesterol). In addition to apoB-100, characteristic of LDL, it contains apolipoprotein (a), a high-molecular-weight protein that resembles plasminogen, covalently binds to apoB-100, and has a high affinity for the vascular wall, thereby promoting cholesterol accumulation. The structure and concentration of Lp(a) vary greatly among individuals, are largely unaffected by diet, and are largely unresponsive to standard lipid-lowering medications. Elevated levels of this lipoprotein are observed in approximately one-fifth of the world's population and are an independent risk factor for atherosclerosis, coronary heart disease, aortic valve stenosis, thrombosis, and stroke.
Several pharmacological approaches to lowering Lp(a) levels are currently being tested: blocking the binding of apolipoprotein(a) to apoB-100 with a small molecule drug (it has successfully completed Phase II trials), post-transcriptional silencing of the LPA gene encoding Lp(a) in the liver, and inactivation of this gene using DNA base editing (this drug has already been administered to the first patients). Zerlasiran (SLN360) belongs to the second approach. It is a drug conjugated to N-acetylgalactosamine (recognized by liver cells) and small interfering RNA (siRNA), which suppresses LPA expression by degrading its messenger RNA. Satisfactory preliminary data on its efficacy and safety were obtained in Phase I clinical trials, paving the way for larger-scale trials.
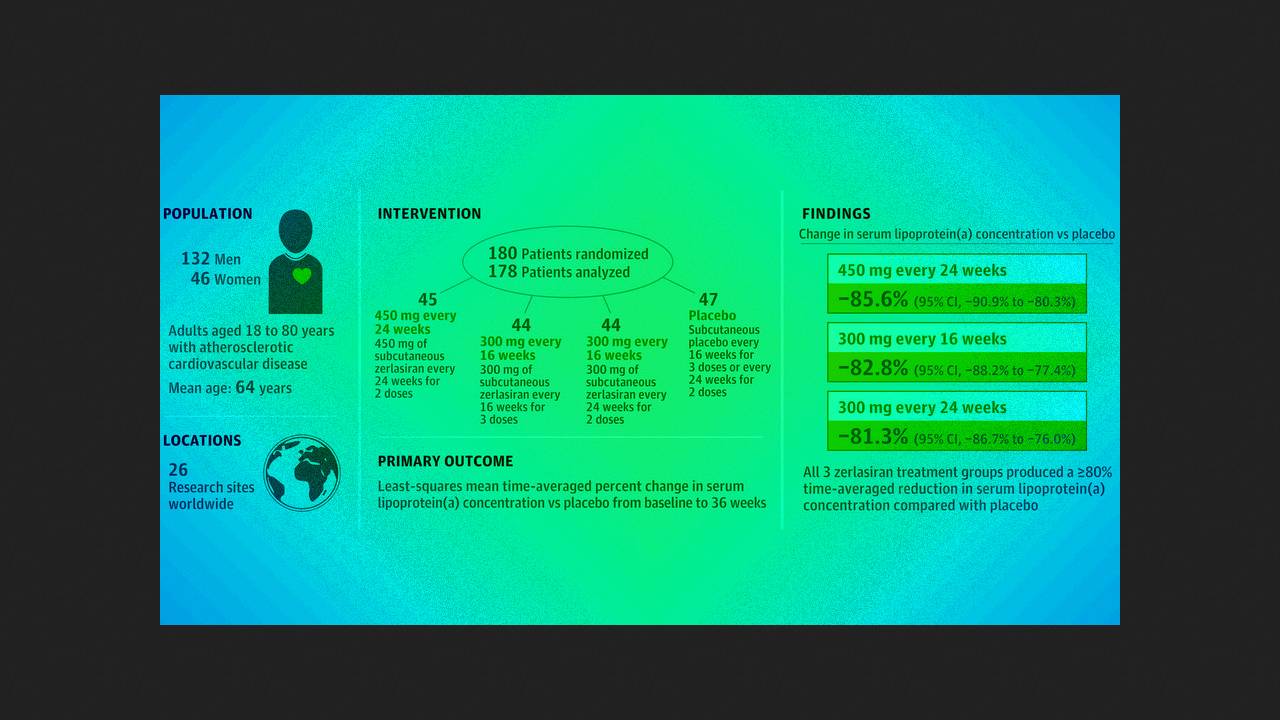
Steven Nissen of the Cleveland Clinic and colleagues conducted the double-blind, randomized, placebo-controlled phase II ALPACAR-360 trial at 26 clinical centers in Europe and South Africa. They enrolled 178 patients (mean age 63.7 years; 25.8 percent women) with serum Lp(a) concentrations of 125 or more nanomoles per liter (mean 213) and stable cardiovascular disease. They were randomly administered subcutaneous 450 milligrams of zerlasiran twice 24 weeks apart, 300 milligrams three times 16 weeks apart, 300 milligrams twice 24 weeks apart, or placebo.
By week 36 of therapy, the time-averaged least-squares reductions in Lp(a) in the active treatment groups, compared with placebo, averaged −85.6, −82.8, and −81.3 percent, respectively, and the median changes were −94.5, −96.4, and −90.0 percent. The most common adverse event was an injection site reaction; mild pain was experienced by 2.3 to 7.1 percent of participants during the first day. During the trial, 20 serious adverse events were reported in 17 patients, all of which were considered unrelated to treatment.
Thus, the miRNA drug zilnasiran, at the tested doses, effectively reduces elevated Lp(a) levels and is well tolerated by patients. Phase III trials are currently underway.
The first miRNA-based drug approved for use was patisiran for the treatment of hereditary transthyrein amyloidosis, and the second was givosiran for the treatment of acute hepatic porphyria. Lumasiran and inclisiran have also been licensed for the treatment of primary hyperoxaluria type 1 and hypercholesterolemia, respectively.