The long-accepted scientific understanding of the oxidative addition reaction on palladium complexes has proven incomplete. Chemists from Germany have discovered that it can proceed not only via a two-electron mechanism but also via a radical mechanism. Moreover, this occurs when both the palladium complex and the substrate are sterically hindered. The study was published in the Journal of the American Chemical Society.
Phosphine complexes of palladium are most often used to catalyze cross-coupling reactions, such as the Suzuki reaction. In this reaction, an organic halogen derivative reacts with an organic boronic acid, forming a carbon-carbon bond and fusing the two organic fragments. Chemists Akira Suzuki, Richard Heck, and Eiichi Negishi received the 2010 Nobel Prize in Chemistry for their discovery of cross-coupling reactions.
The catalytic cycle of almost any cross-coupling reaction begins with the oxidative addition of a halogen derivative to a palladium phosphine complex. During this process, the carbon-halogen bond is cleaved, and its constituents—the halogen and the organic moiety—add to the palladium atom. The mechanism of this reaction has been known since the last century. It is known to proceed as a two-electron concerted process, in which the carbon-halogen bond is cleaved and bonds with palladium are formed, all occurring simultaneously.
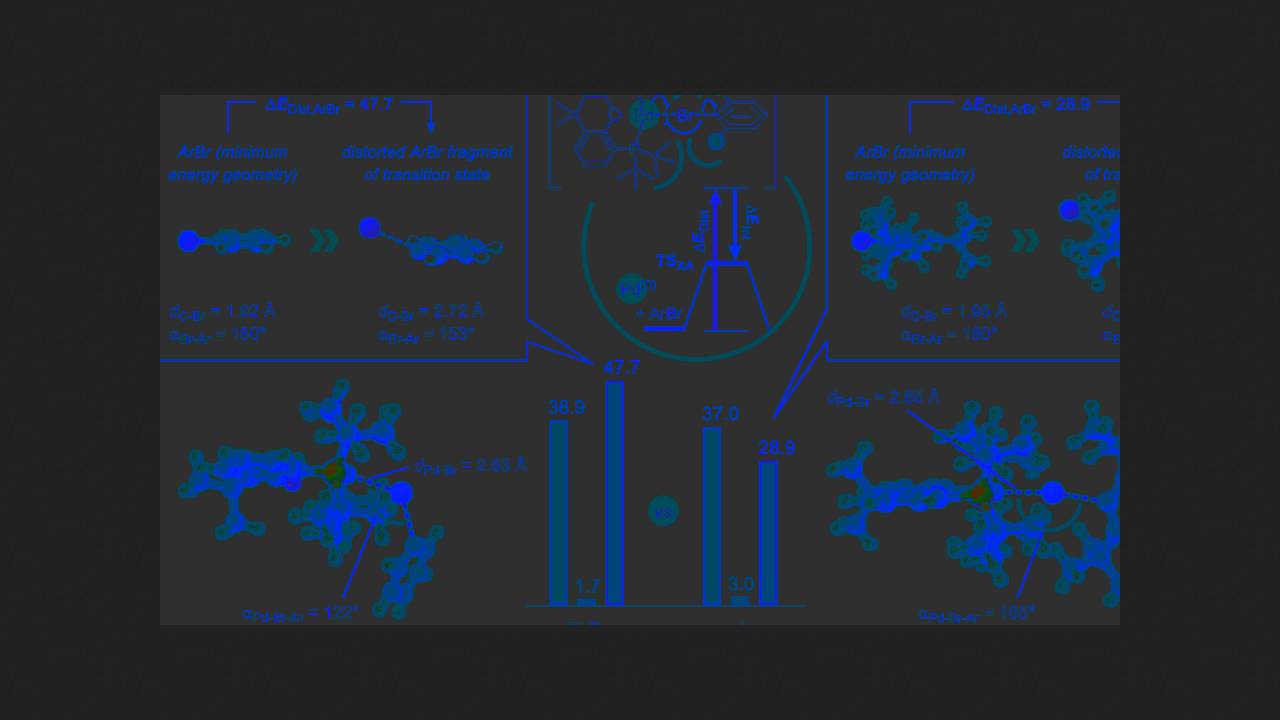
Recently, however, chemists led by Franziska Schoenebeck of the RWTH Aachen University discovered a new mechanism for oxidative addition on phosphine palladium complexes. It turns out that this can proceed radically, with the palladium complex abstracting a halogen atom from the substrate to form an aryl radical.
Chemists discovered this when conducting a cross-coupling reaction with 1-bromo-2-(tert-butyl)benzene, which contains a bulky tert-butyl group next to the bromine atom. Because of this, the reaction simply didn't work with most catalysts—the large tert-butyl group blocked the reaction site from the palladium complex. But to the scientists' surprise, when they used a complex with a very bulky phosphine ligand, the reaction worked. This result didn't match the classical cross-coupling mechanism, and the chemists tried to understand what was going on.
They hypothesized that when the palladium complex's approach to the substrate is severely hindered by surrounding groups, oxidative addition can proceed via a radical mechanism. The scientists then conducted quantum chemical calculations, which showed that this was indeed possible, and that the radical reaction should proceed much faster than the concerted one.
To confirm this experimentally, the chemists used an even more hindered substrate, one with two tert-butyl groups next to the bromine atom. They mixed it with a palladium catalyst and, in just one minute, obtained a product in which the bromine atom was attached to the tert-butyl group. The chemists believe this result confirms the hypothesis of a radical reaction mechanism: the aryl radical formed after the radical oxidative addition abstracted a hydrogen atom from the tert-butyl group, and the resulting new radical attached the bromine atom.
Thus, chemists discovered that oxidative addition reactions can occur even when both the catalyst and substrate are so large that they would seem incapable of reacting with each other. They believe this will expand the possibilities of cross-coupling reactions.
We recently reported on how chemists learned to carry out the Suzuki reaction with anilines.