American and Australian researchers conducted clinical trials and found that muvalapline effectively reduced elevated lipoprotein (a) levels in the blood of patients with high cardiovascular risk. A report on the study was published in the Journal of the American Medical Association.
Lipoprotein(a), or Lp(a), is a type of plasma lipoprotein resembling low-density lipoprotein (LDL), the cholesterol known as "bad" cholesterol. Unlike LDL, it contains, in addition to apoB-100, apolipoprotein(a), a high-molecular-weight protein resembling plasminogen that covalently binds to apoB-100 and has a high affinity for the vascular wall. The structure and serum concentration of Lp(a) vary greatly among individuals, are determined by genetic factors, and are largely independent of diet. Elevated levels are an independent risk factor for atherosclerosis, coronary heart disease, aortic stenosis, thrombosis, and stroke.
Most lipid-lowering drugs are ineffective (there is evidence of moderate efficacy for atorvastatin); high doses of nicotinic acid and, in severe cases, LDL apheresis are typically used. Other agents, including antisense oligonucleopeptides, are also in development. Muvalapline is an orally administered small molecule that blocks the interaction of apolipoprotein (a) with apoB-100, thereby preventing Lp(a) assembly.
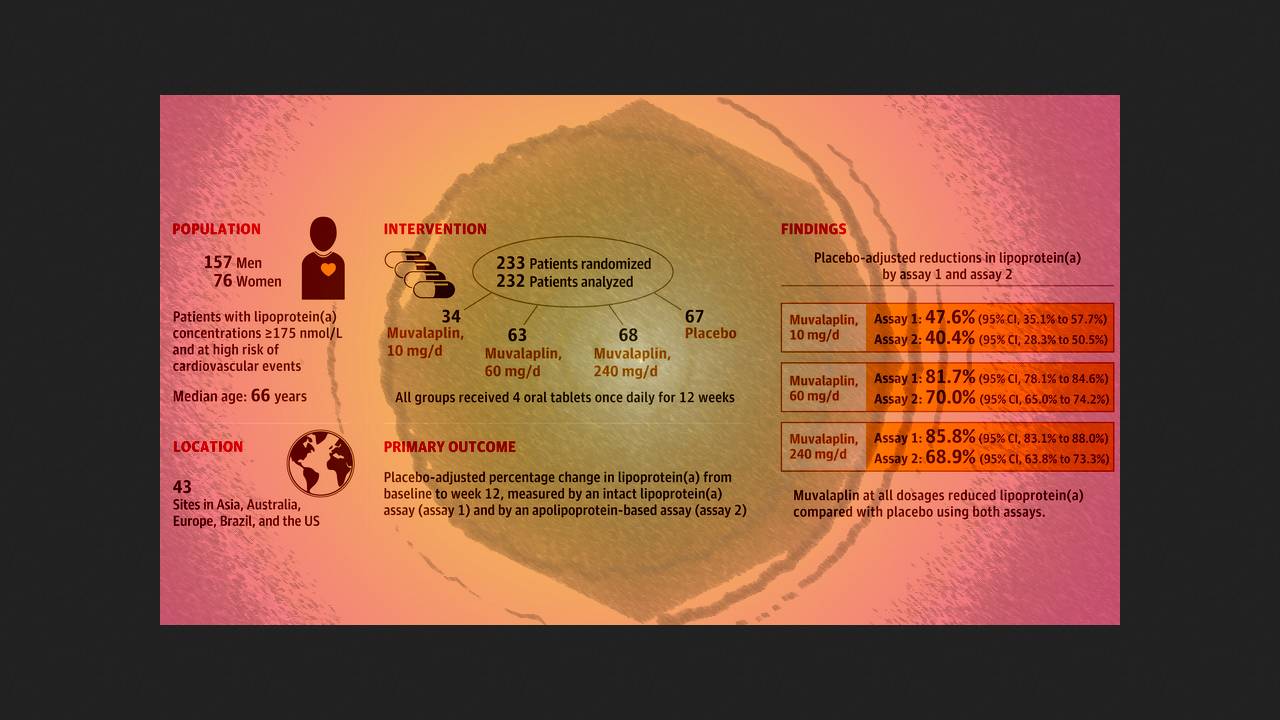
Following the success of the Phase I pilot studies, Stephen Nicholls of Monash University and colleagues in the US conducted a double-blind, randomized, placebo-controlled Phase II trial of KRAKEN in 43 clinics in Asia, Europe, Australia, Brazil, and the US. They enrolled 233 patients (median age 66 years; 33 percent women) with Lp(a) levels of 175 nanomoles per liter or higher and coronary artery disease, diabetes, or familial hypercholesterolemia. They were randomly assigned in a 1:2:2:2 ratio to receive 10, 60, or 240 milligrams of the active drug per day or placebo.
After 12 weeks of treatment, 10, 60, and 240 milligrams of muvalapline resulted in mean reductions in Lp(a) concentrations compared with placebo of 47.6, 81.7, and 85.8 percent for the intact Lp(a) serum assay and 40.4, 70.0, and 68.9 percent for the apolipoprotein(a)-based assay. Active therapy at these doses resulted in Lp(a) levels below 125 nanomoles per liter (an extremely high-risk indicator) in 64.2, 95.9, and 96.7 percent of participants for the former assay and in 38.9, 81.9, and 77.4 percent for the latter. It also resulted in significant reductions in oxidized phospholipids apolipoproteins B and apolipoproteins and LDL-cholesterol.
Muvalapline was well tolerated. The incidence of adverse events was not significantly different between the active treatment and placebo groups and was approximately six percent. These included diarrhea, nausea, respiratory infections, back and muscle pain, uterine leiomyoma, and anemia. No increases in high-sensitivity C-reactive protein (an indicator of inflammation), liver enzymes, or bilirubin were recorded.
Thus, oral administration of mulvalapline for 12 weeks effectively reduced serum Lp(a) levels regardless of the assay type and was well tolerated. Its effect on cardiovascular risk remains to be determined in larger, longer-term trials.
Previously, British and New Zealand researchers reported encouraging results from a clinical trial of DNA base editing for the treatment of heterozygous familial hypercholesterolemia. However, enrollment was subsequently suspended due to serious adverse events in one participant pending clarification of their cause and possible connection with the drug.