American scientists reported the discovery of a new population of neurons that are targets of the adipose tissue hormone leptin. These neurons are neurons in the arcuate nucleus of the hypothalamus that express basonuclein 2. When stimulated by leptin, these neurons directly inhibit AgRP neurons, thereby suppressing appetite in mice. The study was published in the journal Nature.
Leptin, produced by adipose tissue, maintains homeostatic control of adipose tissue mass by regulating food intake and energy balance. This occurs through the inhibition of AgRP neurons and neurons expressing neuropeptide Y, as well as the activation of neurons expressing hypothalamic proopiomelanocortin (POMC neurons). All of these neurons are located in the arcuate nucleus of the hypothalamus.
In general, two neuronal populations—AgRP neurons and POMC neurons—antagonistically regulate leptin-mediated food intake. However, the functional effects and process dynamics of these neurons differ in several important respects, and several lines of evidence suggest the existence of other leptin-sensitive neuronal populations that may be critical for leptin-mediated control of food intake and body weight.
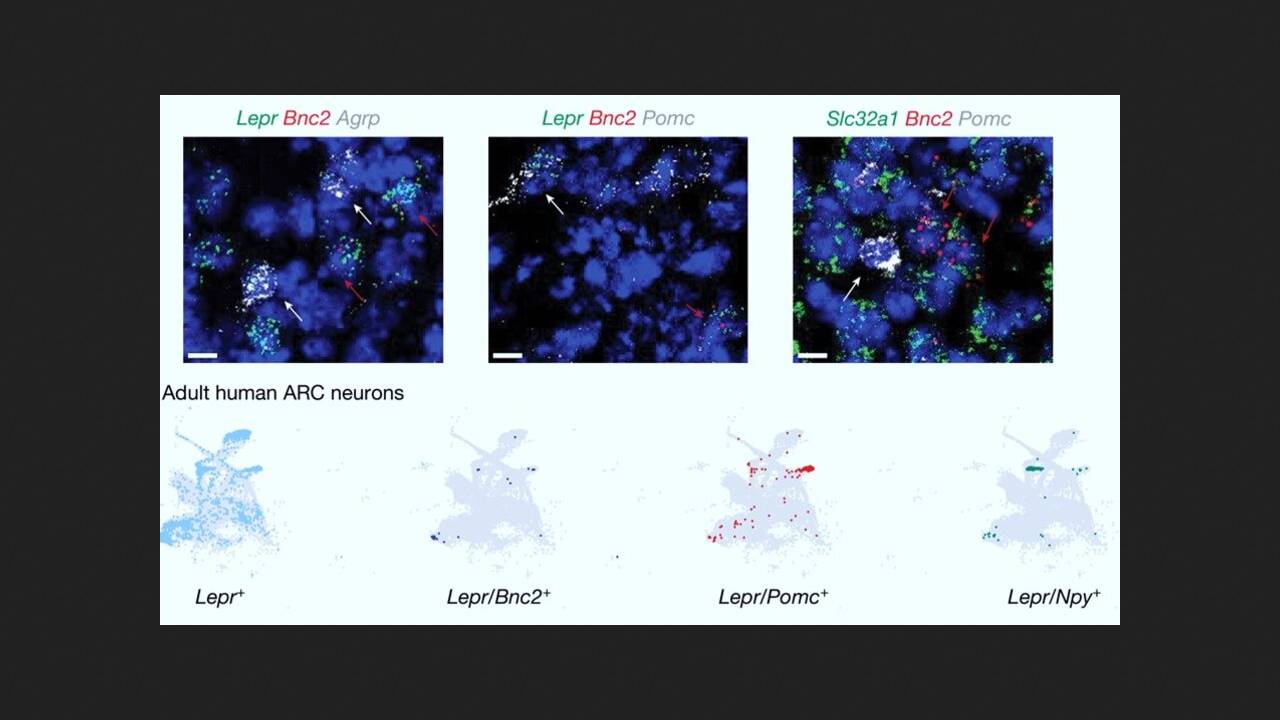
Researchers led by Jeffrey Friedman of Rockefeller University systematically profiled the transcriptomes of neurons in the arcuate nucleus of the hypothalamus in mice using single-nucleus RNA sequencing. It was discovered that one cluster contained previously undescribed neurons expressing the leptin receptor. These neurons were identified as expressing basonuclin 2 (BNC2 neurons), a basonuclin-zinc finger protein involved in the regulation of mRNA splicing, processing, and transcription, and play a key role in early embryonic development.
To further study the dynamics and function of BNC2 neurons, the scientists bred a line of mice with a knockout of the BNC2 gene. Experiments showed that BNC2 neurons respond to sensory cues associated with food depending on experience, and that food consumption further activates these neurons. In the experiment, mice that had fasted overnight were given food for two or ten minutes. After the food was removed, BNC2 neuron activity rapidly declined, but remained high with constant access to food.
Further molecular studies revealed that some of the sensory signals that suppress AgRP neurons and reduce appetite after a meal are transmitted by BNC2 neurons. Subsequently, several experiments demonstrated that leptin increases the activity of BNC2 neurons. These neurons, in turn, directly inhibit the activity of AgRP neurons, leading to appetite suppression.
Deletion of leptin receptors in BNC2 neurons caused excessive appetite and led to obesity in mice. Similar changes were observed with knockout of leptin receptor genes in AgRP neurons. Notably, the scientists also observed improved glucose tolerance and insulin sensitivity in mice after activation of BNC2 neurons.
Thus, the scientists conclude that the population of BNC2 neurons in the arcuate nucleus of the hypothalamus directly and rapidly regulates feeding and energy balance. These results add a new important component to the neural circuitry responsible for appetite and its disorders and contribute to our understanding of the mechanisms by which leptin regulates this system. Potentially, pharmacological activation of these neurons may have therapeutic value for weight loss.
We previously reported that the taste and smell of food induce mitochondrial fragmentation in the liver through the activation of POMC neurons.