Spanish researchers performed a multi-omics analysis of individual cells in an infusion product of therapeutic chimeric antigen receptor T cells and found that the composition and properties of the cells, as well as their dynamics after administration, significantly impact treatment efficacy. Their report was published in the journal Cell Reports Medicine.
Chimeric antigen receptor T cells (CAR-T cells) are enabling radical advances in cancer therapy, including complete cures (for more information on this technology, see the article "Chimera vs. Cancer"). Currently, drugs for the treatment of B-cell hematologic malignancies and a type of synovial sarcoma have entered clinical use. These drugs are effective in approximately half of patients who have failed other therapies, while the other half show an insufficient or no response. The likelihood of success depends on a variety of factors, which are currently being studied.
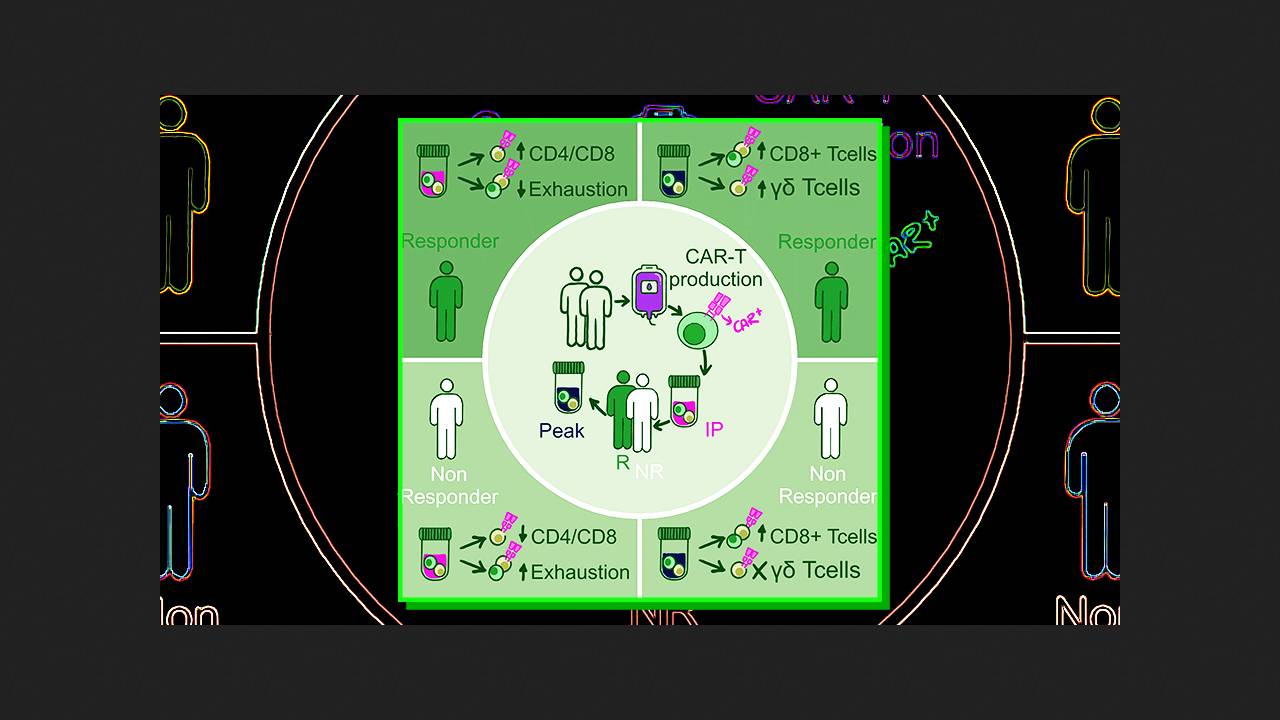
Pablo Menéndez of the Josep Carreras Leukemia Research Institute and colleagues conducted an integrated analysis of the clonal kinetics and transcriptomic heterogeneity of CD19-CAR T-cell infusion products (varnimcabtagene autoleucel, varni-cel) manufactured ex vivo for five patients with refractory or relapsed B-cell acute lymphoblastic leukemia. They performed αβ T-cell receptor and RNA sequencing in individual cells isolated by flow cytometry based on CAR expression. The cellular dynamics analysis included both CAR-transduced (CAR-positive) and non-transduced (CAR-negative) T-cells in the product.
The CAR transduction rate in the infusion products ranged from 23 to 38 percent. CAR T-lymphocyte proliferation rates varied across patients, peaking between the first and fourth weeks after infusion. Two patients experienced early relapses in the second and third months, one experienced a late relapse in the sixth month, and two achieved a sustained complete response to therapy. A total of 37,100 single high-quality T-lymphocytes were analyzed across all samples at various stages. Clusters of naive CD4+ cells, effector, memory, and cytotoxic CD8+ cells, and cytotoxic γδ T-cells were clearly visible.
A comparison of CAR-positive and CAR-negative cell subtypes in the infusion product revealed that the former proliferate significantly more actively, while the non-proliferating fraction of the latter contains a significantly higher proportion of effector CD8+ cells. Patients with a complete response to therapy were found to have at least three times more CAR-positive CD4+ cells in the infusion product than CD8+ cells. The impact of this ratio on efficacy was confirmed (p = 0.03) in a separate cohort of 47 patients with B-cell acute lymphoblastic leukemia. Moreover, the expression of markers of T-lymphocyte depletion preceding transduction negatively correlated with treatment success.
At the peak of proliferation, the proportion of proliferating CAR-positive and CAR-negative T cells in patients' bodies decreased, being replaced by CD8+ cells, which predominated over CD4+ cells among non-proliferating CAR-positive cells. At the same time, the population of both positive and negative γδ T cells increased significantly at the peak, and its growth directly correlated with therapy efficacy. The clinical significance of these cells was confirmed in retrospective cohorts of 18 patients with acute B-cell lymphoblastic leukemia treated with varnicel and 58 patients with diffuse large B-cell lymphoma treated with axicel or tisacel.
The obtained results indicate the complex nature of the T-cell response in CD19-CAR-T therapy, which is not limited to CAR-positive αβ T cells. In particular, the CD4+/CD8+ cell ratio in the infusion product, prior cell depletion, and the expansion of γδ T cells after infusion are of great importance. Influencing these factors may potentially improve treatment success, the authors conclude.
Previously, American researchers were able to significantly increase the effectiveness of CAR-T therapy in preclinical trials on mice using an experimental modified human interleukin-7, which stimulates lymphocyte growth.