US chemists have synthesized a covalent organic framework capable of selectively absorbing carbon dioxide from the air. When this substance was heated, the carbon dioxide was desorbed back into the air. As the study's authors report in the journal Nature, the resulting material withstood more than 100 adsorption-desorption cycles of carbon dioxide from outdoor air.
Covalent organic frameworks (COFs) are crystalline and porous structures constructed from organic molecules. They are often used for the selective absorption of gases, including carbon dioxide (CO2). However, chemists have not yet developed frameworks capable of selectively and reversibly absorbing CO2 from the air because its concentration in the air is very low—approximately 400 parts per million.
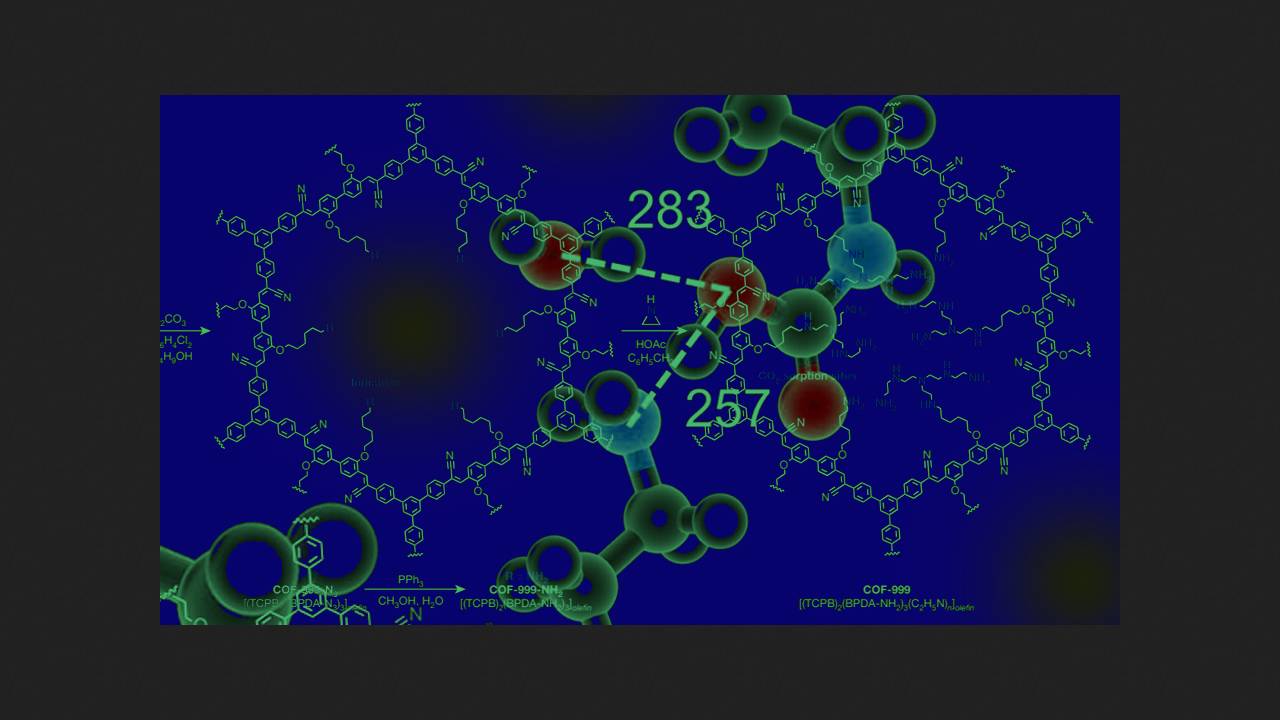
Recently, however, scientists led by Omar M. Yaghi of the University of California, Berkeley, developed a framework capable of selectively absorbing CO2 from the air. Their idea was to fill the framework's pores with amino groups capable of covalently bonding carbon dioxide molecules into carbamates.
To obtain the desired structure, the chemists first synthesized a framework with a large number of azide groups from modified biphenyldicarbaldehyde and 1,3,5-tris(4-cyanomethylphenyl)benzene, which underwent a Knoevenagel condensation. The scientists then reduced the resulting compound with triphenylphosphine in water, converting the azide groups into amino groups. The chemists then mixed the product of this reaction with aziridine, which was opened by the framework's amino groups to form branched carbon chains containing numerous new amino groups. This yielded the polyamine framework COF-999, which they characterized using solid-state NMR spectroscopy.
The chemists then tested COF-999's ability to adsorb various air components—nitrogen, oxygen, argon, and carbon dioxide. They found that the first three were virtually unabsorbed by the framework, but carbon dioxide was absorbed rapidly and in large quantities. At zero humidity, COF-999 adsorbed up to 0.96 millimoles of carbon dioxide per gram of framework. And with increasing humidity to 50 percent, the material's capacity increased to two millimoles of CO2 per gram.
The researchers then tested COF-999 in outdoor conditions. Over the course of 20 days, the scientists conducted 100 consecutive adsorption-desorption cycles of CO2 from Californian outdoor air. On average, the scaffold's capacity was 1.28 millimoles of CO2 per gram. Moreover, the structure and activity of COF-999 remained unchanged after the 20-day experiment.
Thus, chemists synthesized the first covalent organic framework capable of selectively absorbing up to two millimoles of carbon dioxide per gram of absorbent within an hour. In the future, the researchers plan to synthesize a set of similar frameworks and compare their CO2 absorption capabilities.
Previously, we talked about how chemists synthesized a covalent organic framework with topological bonds.