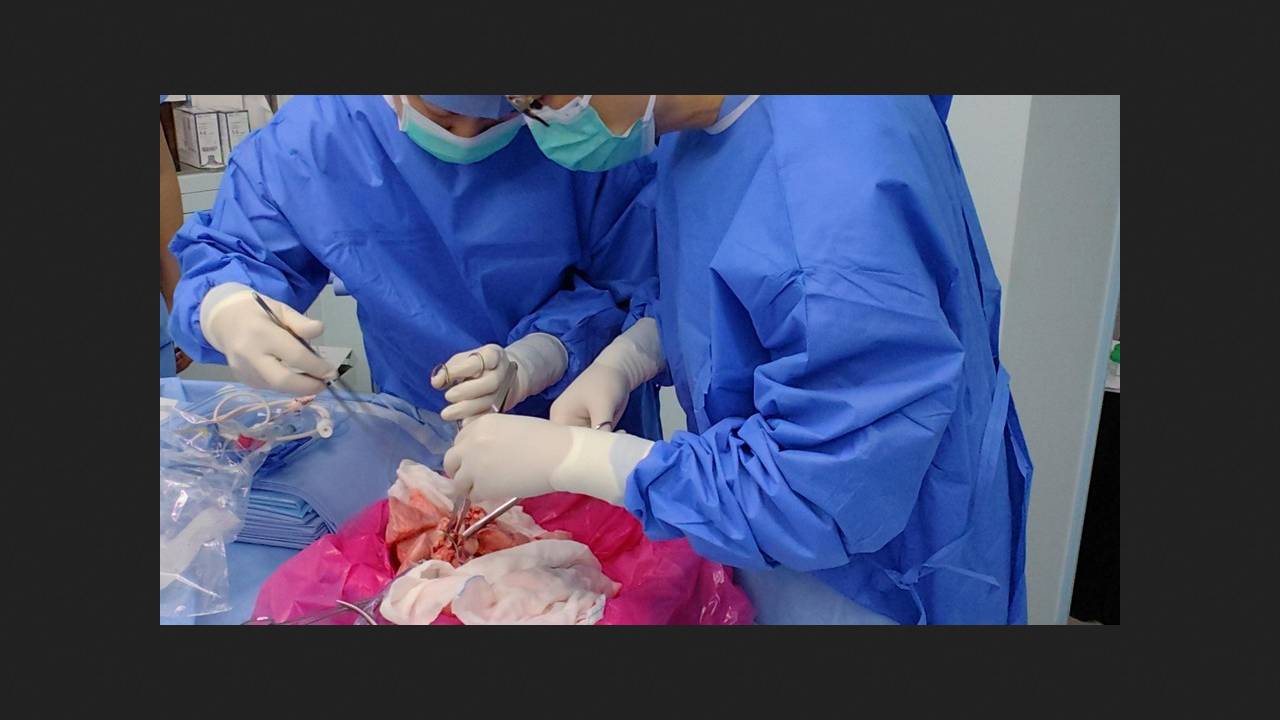
Chinese researchers have performed the world's first transplant of a lung from a genetically modified pig into a patient confirmed to be brain dead. The organ remained viable and functional throughout the nine-day experiment, although complications were not avoided. The article was published in the journal Nature Medicine.
The idea of transplanting animal organs into humans (xenotransplantation) has been around for a long time, but genome editing systems, including CRISPR-Cas, have paved the way for its practical application. These systems can be used to remove key animal antigens, insert human genes into the genome, and inactivate embedded viruses to improve tissue compatibility with the human body.
The first experiments with transplanting genetically modified pig kidneys and hearts were conducted on brain-dead patients; since then, both organs have been transplanted into living people in the United States. In May 2025, Chinese researchers reported the first transplant of a GM pig liver, in addition to their own, into a brain-dead patient with liver failure. The organ functioned for 10 days and was then routinely removed. The lung is a more challenging organ to transplant, as it is easily damaged and exposed to the surrounding air (which increases the risk of infection), and similar experiments have not yet been conducted.
To prepare for the transplant, the Chinese company Clonorgan Biotechnology introduced six modifications into the genome of a Bama Xiang pig: they knocked out the GGTA1, B4GALNT2, and CMAH genes, which are responsible for xenoantigen production, and inserted the human genes CD55, CD46, and TBM, which regulate the complement system and modulate the immune response. Xin Xu of Guangzhou Medical University and colleagues harvested the left lung from a 22-month-old, 70-kilogram male GM pig and prepared it for transplant.
The recipient was a 39-year-old man with confirmed brain death following a hemorrhage who could not donate organs due to contraindications. The surgery was performed via a median sternotomy, with a cold ischemia time of 206 minutes. Immunosuppression with rabbit thymoglobulin, mycophenolate mofetil, and tacrolimus was initiated the day before the surgery. The latter two agents, along with methylprednisolone, were administered daily thereafter. Postoperative immunosuppression and anti-inflammatory therapy also included basiliximab, rituximab, eculizumab, tofacitinib, and belatacept.
The patient underwent continuous monitoring of physiological, biochemical, and immunological parameters, and graft biopsies were performed. After reperfusion, the transplanted lung remained viable, expressed human transgenes, and functioned, with a fractional oxygen uptake of 40 percent. Radiographs at 6 and 24 hours showed decreased lung tissue transparency. A CT scan 24 hours after the procedure revealed a consolidation in the lower lung, consistent with primary graft dysfunction (possibly due to reperfusion injury). Histologically, severe inflammatory edema with CD68-positive cell infiltration and elevated levels of interleukin 6 and 10 was detected. B- and NK-cell levels fluctuated in the early postoperative period, while T-cell levels gradually increased beginning on the third day.
On days three and six after the procedure, immunoglobulin G deposits were observed along the alveolar septa in the xenograft; by day nine, these deposits had significantly decreased. Immunoglobulin M levels were initially low but increased by day nine, indicating secondary immune activation. Significant complement activation was also observed beginning on day three. There were no signs of active infection, including those caused by porcine pathogens. The graft remained viable for 216 hours of the experiment; acute rejection did not occur, and hemodynamic parameters remained stable. After the planned period, the recipient was disconnected from life support.
A pilot experiment demonstrated the fundamental feasibility of xenotransplanting a genetically modified pig lung without acute rejection. However, despite the modifications, a pronounced immune response, both cellular and humoral, was observed. This suggests the need to refine the immunosuppressive and anti-inflammatory therapy protocol, primarily by incorporating blockade of the CD40 signaling pathway and suppression of interleukins and the complement system.
We wrote about xenotransplantation in detail in the article "About Pigs and People."