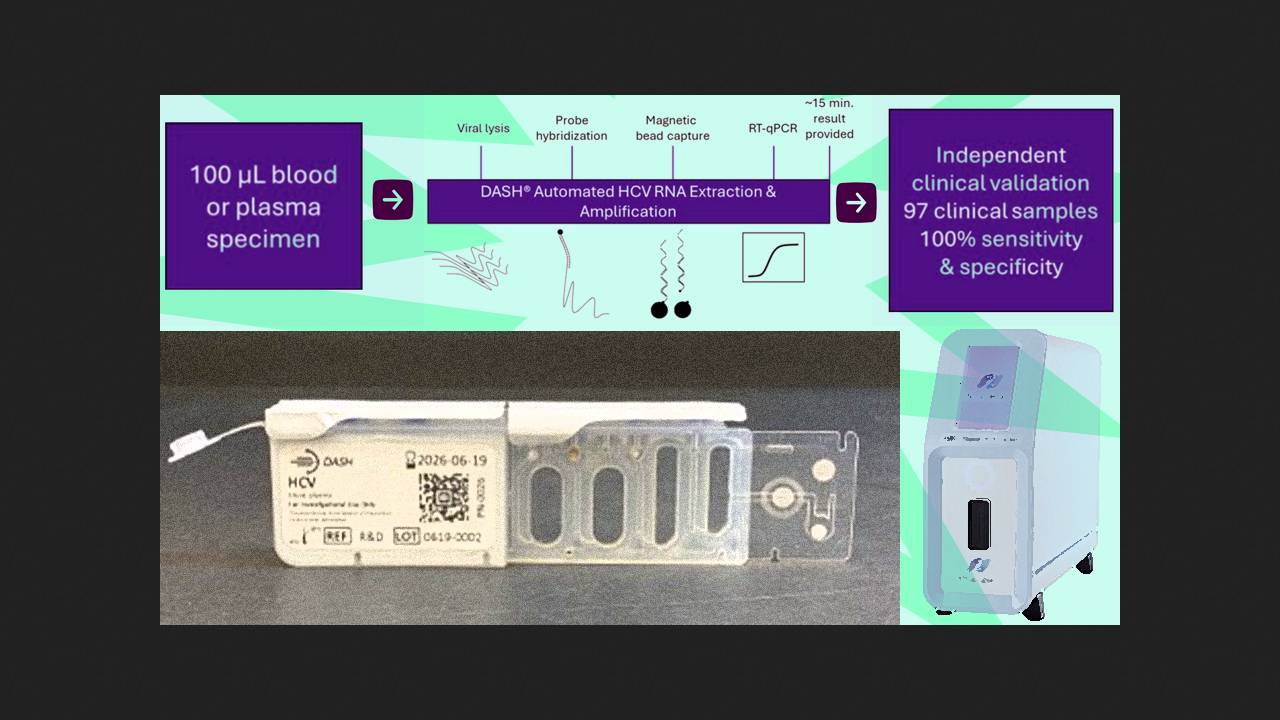
Sally McFall of Northwestern University in Chicago and her colleagues have developed and tested a new diagnostic system for point-of-care detection of hepatitis C. The test delivers accurate results in 15 minutes, significantly faster than all existing tests. This timeframe allows for a result to be obtained during a single doctor's appointment, and, if the result is positive, immediate treatment can be initiated. A report on the development was published in The Journal of Infectious Diseases.
The analytical cartridge for hepatitis C diagnostics is designed for use with the portable DASH test system, previously developed at Northwestern University for rapid diagnostics of COVID-19 and influenza. A 100-microliter sample of whole blood or plasma is sufficient for analysis. The system automatically lyses viral particles, hybridizes the sample, captures it on magnetic particles, and performs high-quality real-time PCR. It recognizes 1–6 viral genotypes with a detection limit of 200 international units per milliliter. After testing on various biological samples and standardized viral samples, the analytical system was submitted for independent testing on 97 biological samples at Johns Hopkins University, where it demonstrated 100% agreement between positive and negative results with existing clinical assays.