German chemists synthesized and characterized several halogenated silylium salts with a carborane moiety as the anion. They were found to be stronger Lewis acids than alkylated silylium salts, but they gradually decompose in solution. The study was published in the journal Nature Chemistry.
Many reactions involving halosilanes produce silylium salts as intermediates—ionic species with a cation containing trivalent silicon. For a long time, chemists were unable to obtain this salt in pure form, but in 2002, scientists finally succeeded in reliably characterizing a silylium salt for the first time. It consisted of a trimethylsilyl cation and a noncoordinating carborane anion. Since then, chemists have been attempting to produce silylium salts in which silicon is bound not only to carbon atoms but also to atoms of other elements.
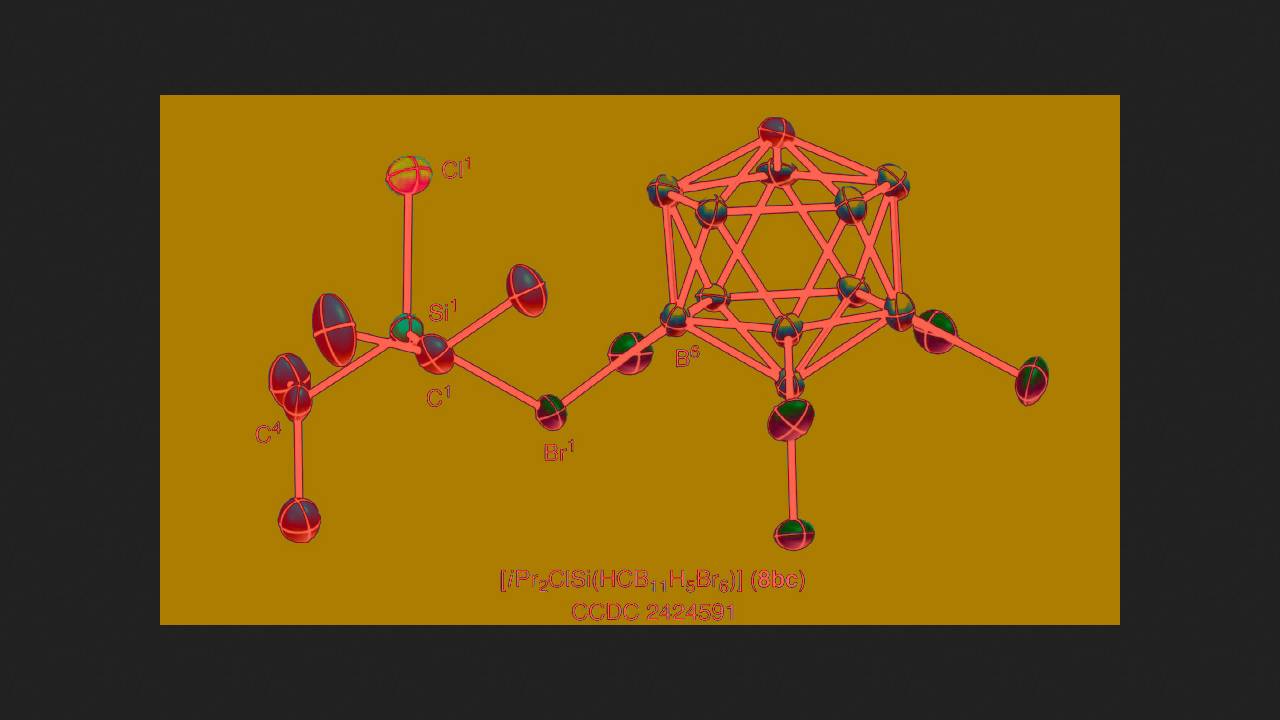
Martin Oestreich and his colleagues from the Technical University of Berlin decided to attempt to produce halogenated silylium salts. To do this, they took diisopropylchlorosilane, in which two isopropyl groups, a hydrogen atom, and a chlorine atom are attached to the silicon atom, and mixed it with a carborane salt of a triphenylmethyl cation in deuterobenzene. The scientists' idea was that the cation would abstract a hydrogen atom from the silane, forming the desired chlorine-substituted salt. Instead, the researchers observed the formation of a salt with a different cation, in which two isopropyl groups and a hydrogen atom were attached to the silicon.
They then decided to use a different synthesis method: the chemists mixed the starting silane with a salt in which protonated benzene served as the cation. In this case, the reaction proceeded as expected—hydrogen was released, forming a salt with a cation containing a silicon-chlorine bond and two isopropyl groups. The chemists characterized it using NMR spectroscopy and X-ray diffraction analysis.
Using the same method, scientists obtained fluorine-, bromo-, and iodine-substituted silylium salts. These were stable in solid form under an inert atmosphere, but gradually decomposed in solution, decomposing especially rapidly in light.
The chemists then decided to test the strength of the resulting silylium salts as Lewis acids, that is, their affinity for lone electron pairs. To do this, the scientists used NMR spectroscopy of the adducts of their salts with fluorinated benzonitrile, as well as quantum chemical calculations. It turned out that, of all previously obtained silylium salts, the halogenated salts are the strongest Lewis acids.
Thus, scientists synthesized halogenated silylium salts for the first time and studied their acidic properties. According to the authors of the article, the resulting compounds are among the strongest Lewis acids ever isolated in pure form.
Organosilicon compounds, including silanes, are most often used to produce polymers. Recently, chemists in France have learned to convert these polymers back into monomeric chlorosilanes, specifically dimethylchlorosilane.