American chemists obtained an N-heterocyclic carbene that was stable in aqueous solution. It was not protonated by water and did not react with nucleophiles due to its bulky carborane substituents. The study was published in Science Advances.
Carbenes are organic compounds with a divalent carbon atom in their structure. This carbon atom typically has a lone electron pair and an unoccupied orbital, making carbenes highly reactive toward nucleophiles and electrophiles. N-heterocyclic carbenes are more stable than others due to the electronic effects of the two nitrogen atoms located near the carbene center. However, even they quickly decompose in air.
Chemists led by Vincent Lavallo of the University of California, Riverside, recently showed that N-heterocyclic carbenes can be stable not only in air, but even in aqueous solution.
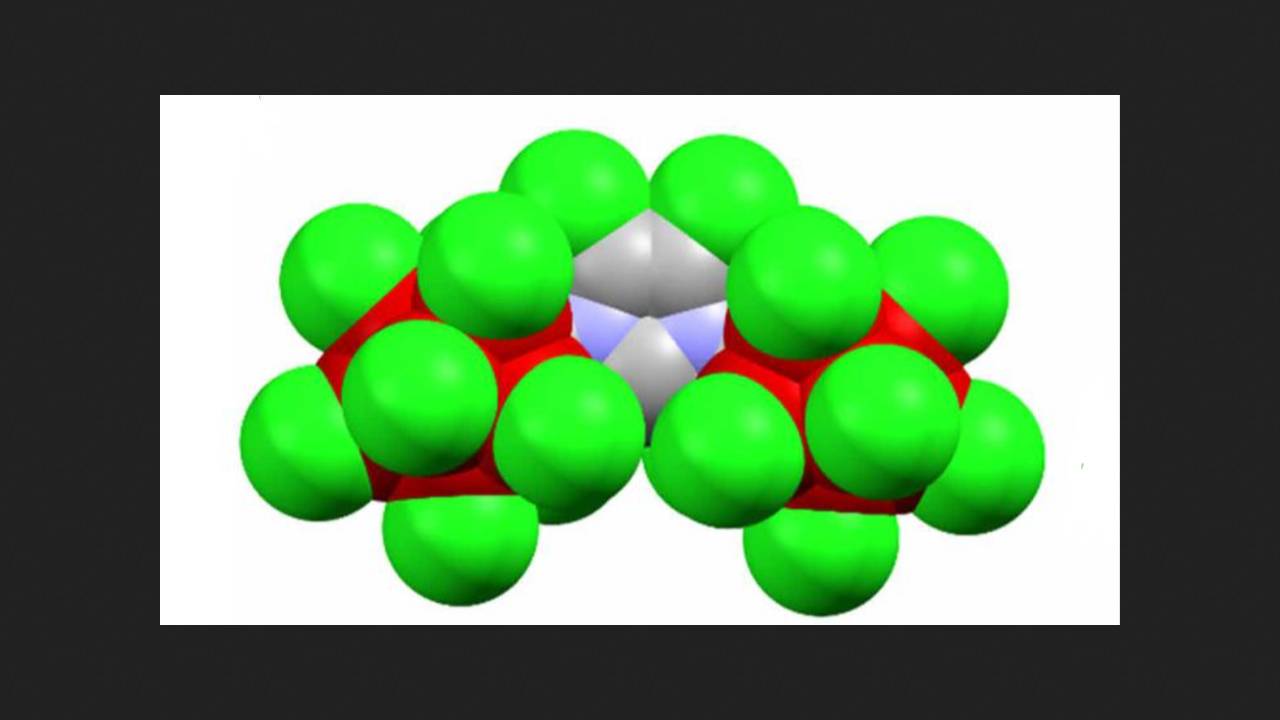
To obtain this carbene, scientists introduced two bulky cluster substituents (carborane) into the N-heterocyclic carbene precursor molecule. These substituents contained nine boron atoms and one carbon atom. Each boron atom formed a bond with other boron atoms, and the single carbon atom in the cluster attached to the carbene fragment. Next, to increase the stability of the resulting carbene, the chemists chlorinated these substituents. Now, each boron atom was bonded to a chlorine atom.
The chemists mixed the resulting chlorinated precursor with lithium hexamethyldisilazide, a strong base. This formed an N-heterocyclic carbene. The scientists characterized it using NMR spectroscopy and X-ray diffraction analysis. During their work, they noticed that if a loosely sealed NMR ampoule containing the carbene solution was left exposed to air, its NMR spectrum remained unchanged—that is, the carbene did not decompose. They then added distilled water to the carbene solution in tetrahydrofuran. To their surprise, even after adding water, the NMR spectrum remained unchanged. Thus, they discovered that the resulting carbene was stable in aqueous solution.
The scientists then demonstrated that their carbene is not only stable in water but can also be obtained in aqueous solution. They mixed the carbene precursor and lithium hydroxide in a mixture of water and tetrahydrofuran, resulting in pure carbene with no signs of decomposition.
Thus, chemists obtained the first water-stable carbene. Moreover, it did not react not only with water but also with complexes of various transition metals, heterocumulenes, and elemental selenium. The authors of the article suggest that the low reactivity of the resulting carbene is due to the electron-withdrawing and large carborane substituents, which "protect" the carbene from other molecules.
Previously, we talked about how chemists first obtained crystalline nitrene that was stable at room temperature.