Australian, American, British and Dutch investigators reported on the successes of another phase of clinical testing of a drug based on small interfering RNA in hyperthyroidism lipoprotein (a). A report about the robot was published in the Journal of the American Medical Association.
Lipoprotein (a), or LP (a) is a whole blood plasma lipoprotein, which is called low-density lipoprotein (LDL, the reservoir of “bad” cholesterol). The cream of apoB-100, characteristic of LPNG, contains apolipoprotein (a) - a high-molecular protein, which is called plasminogen, covalently binds to apoB-100 and has a high affinity to the vessel wall, This removes the accumulated cholesterol in it. The structure and concentration of lipid(a) vary greatly in different people, they are practically not present in children and do not respond to standard lipid-reducing tests. The rise of this lipoprotein affects approximately one-fifth of the world's population and is an important factor in the high risk of atherosclerosis, ischemic heart disease, aortic valve stenosis, thrombosis and stroke.
Currently, a number of pharmacological approaches are being tested to reduce the level of LP(a): blockade of the binding of apolipoprotein(a) to apoB-100 with a low-molecular-weight drug (which has successfully passed another testing phase) , post-transcriptional silencing of the encoding LP(a) gene LPA in the liver, as well as inactivation of this gene due to additional editing of DNA bases (such a drug was already administered to the first patients. Zerlasiran (SLN360) should be used before another approach. This drug is used conjugated with N-acetylgalactosamine (recognized by liver cells) small interfering RNA (siRNA), which inhibits the expression of LPA by degrading its messenger RNA. phase provided sufficient early data on the effectiveness and safety that allowed for larger-scale testing.
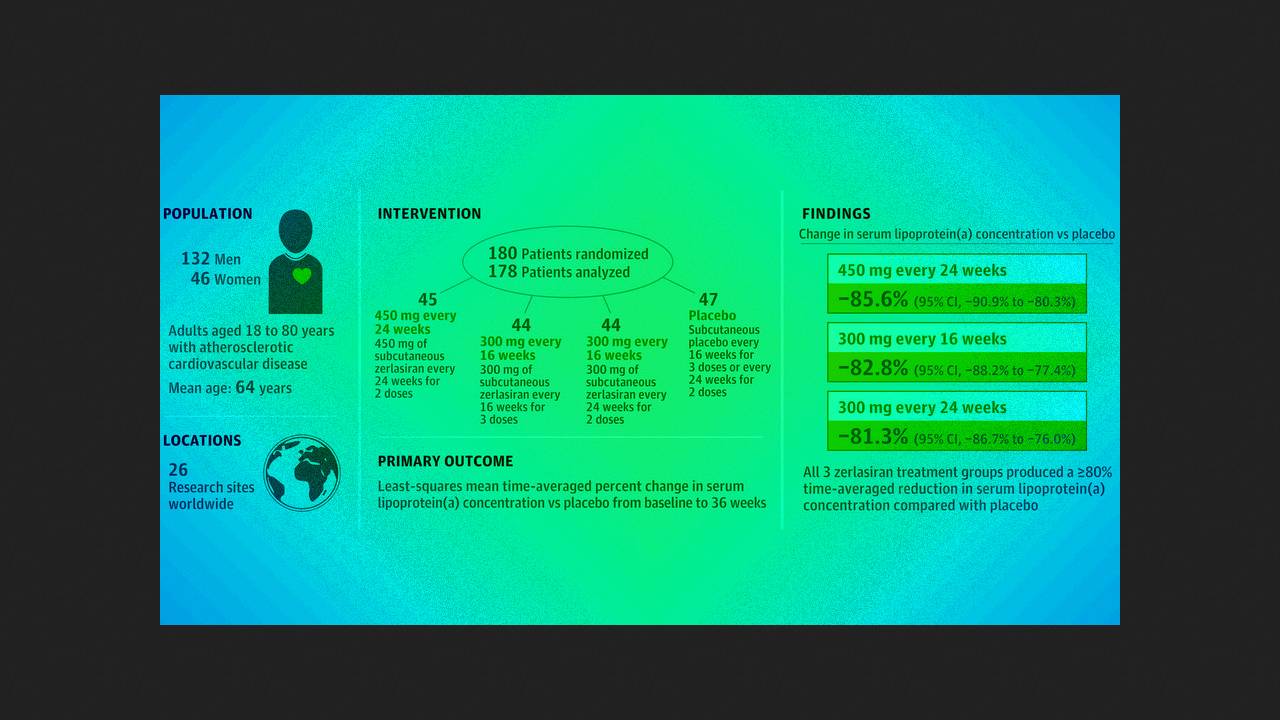
Steven Nissen of the Cleveland Clinic and colleagues conducted a double-sleeve, randomized, placebo-controlled trial of another phase of ALPACAR-360 at 26 clinical centers in Europe. Deep Africa. They studied 178 patients (middle age 63.7 people; 25.8 people - women) with a serum concentration of Lp(a) of 125 or more (on average 213) nanomoles per liter and stable heart-related illnesses. Vipadkovo was administered subcutaneously or 450 milligrams of zerlasiran twice at an interval of 24 days, 300 milligrams three at an interval of 16 days, 300 milligrams two at an interval of 24 days or placebo.
Up to the 36th stage of therapy in the active therapy groups, the averaged hourly decrease in the level of Lp(a), calculated by the method of least squares, compared with placebo became an average of −85.6; −82.8 and −81.3 per cent, and the median change in this indicator was −94.5; −96.4 and −90.0 hundred. The most common side effect was a reaction at the injection site, which was observed in 2.3 to 7.1 hundred participants during the first day. Within an hour of testing, 20 serious adverse reactions were recorded in 17 patients, all of which were found to be unrelated to treatment.
Thus, the miRNA drug zilnasiran in the tested doses effectively reduces the level of LP(a) and is well tolerated by patients. The third phase will now be tested.
The first to praise the drug based on miRNA was patisiran for the treatment of congestive transthyrein amyloidosis, the other was givosiran for the treatment of acute hepatic porphyria. Also, until now, lumasiran and inclisiran are licensed for the treatment of primary hyperoxaluria type 1 and hypercholesterolemia type 1.