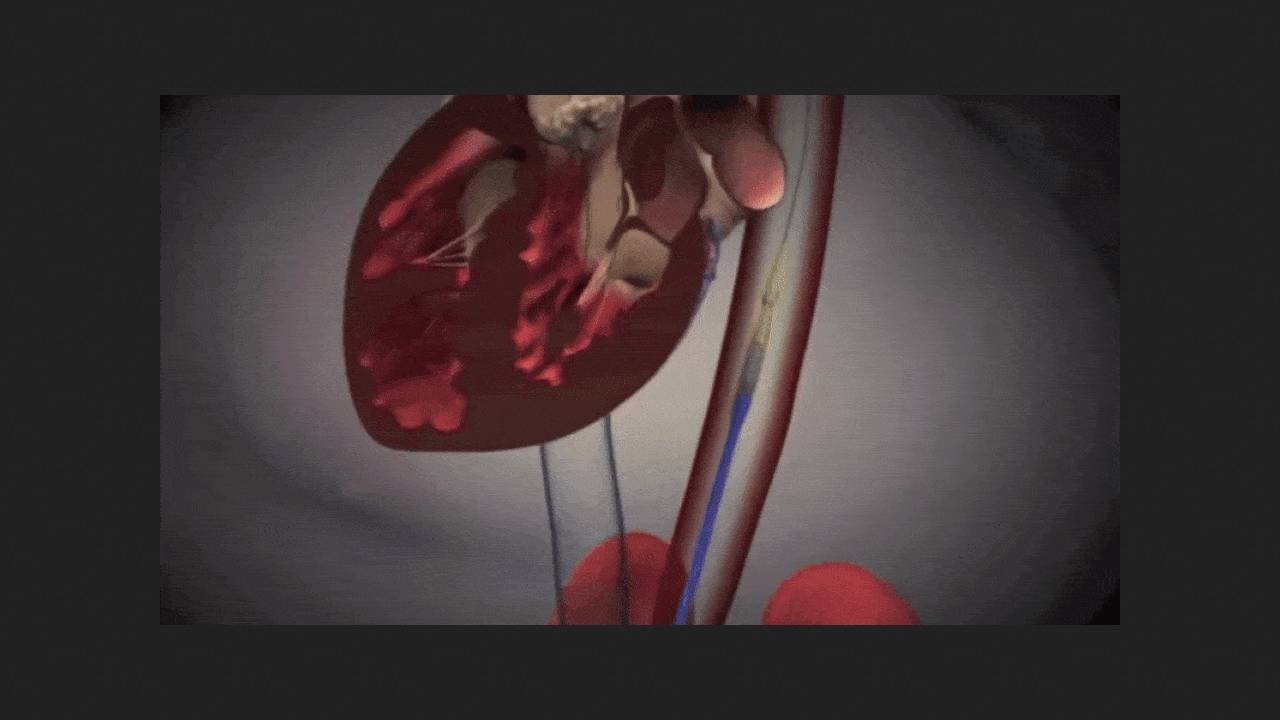
The US Food and Drug Administration (FDA) has granted the first approval for transcatheter aortic valve replacement (TAVR) for asymptomatic severe aortic stenosis. The SAPIEN 3 system from Edwards Lifesciences was licensed. As the company's press release emphasized, without treatment, one in ten patients with symptoms of severe aortic stenosis dies within five weeks of their onset. Symptoms can develop unexpectedly and progress rapidly and unpredictably. However, until now, TAVR has only been indicated in patients with clinically evident disease.
The decision was prompted by the results of the multicenter, randomized, controlled clinical trial EARLY TAVR, which enrolled 901 patients with asymptomatic severe aortic stenosis. Of these, 455 underwent TAVR, while the remaining 446 were kept under dynamic observation according to current guidelines. During a median follow-up period of 3.8 years, death, stroke, or unplanned hospitalization for cardiovascular causes occurred in 26.8 percent of participants in the study group versus 45.3 percent in the control group (p < 0.001).