British chemists obtained a 48-carbon ring in solution. To do this, they wrapped three other rings around the carbon ring to prevent the cyclocarbon molecules from reacting with each other. The resulting cyclocarbon was stable in solution at low temperatures but decomposed instantly upon evaporation of the solvent, the study's authors write in Science.
In cyclocarbon molecules, the carbon atoms are linked into a ring by multiple bonds. These compounds contain no other atoms, so they can be considered a separate allotrope of carbon. In solution or solid phase at room temperature, these substances are unstable and decompose quickly, so they are usually obtained on substrates at temperatures below 10 Kelvin.
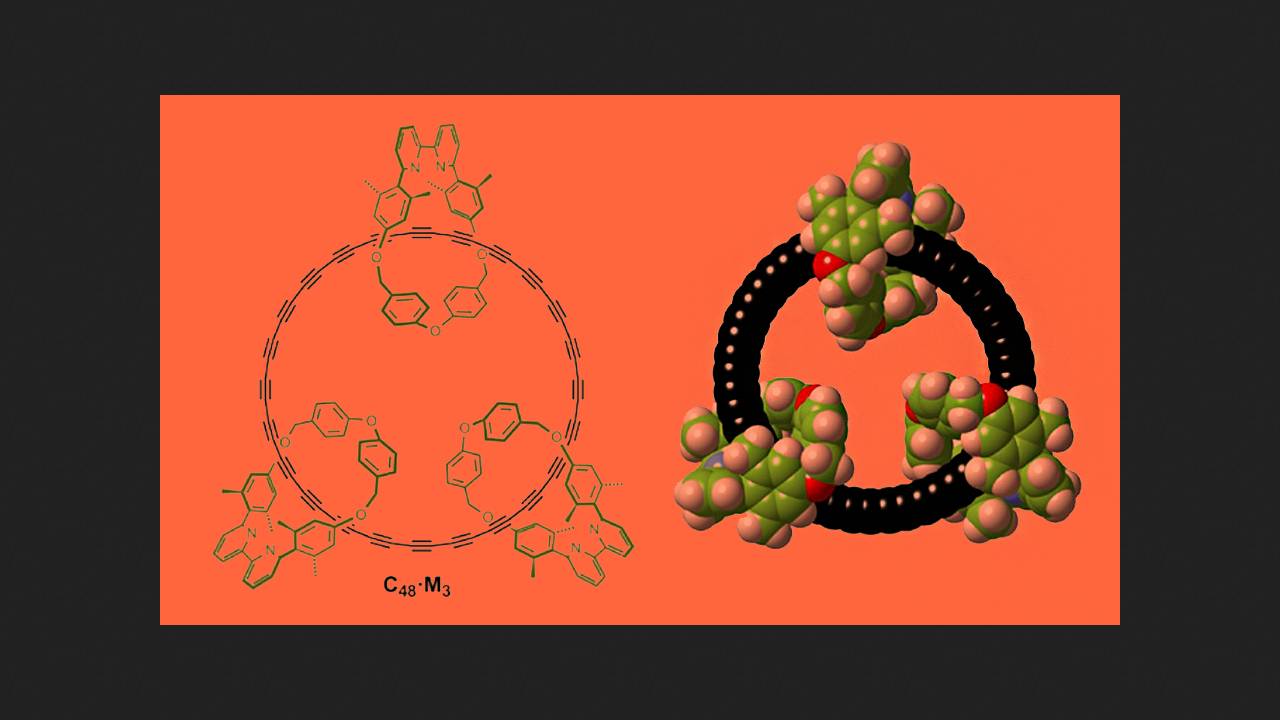
Chemists led by Harry L. Anderson of Oxford University decided to obtain cyclocarbon in solution, despite its instability. The scientists' idea was to synthesize a cyclocarbon derivative in which several cyclic organic molecules would be wrapped around the carbon chain. The chemists hypothesized that these molecules would protect the carbon chain from collisions with neighboring molecules and, consequently, from rapid decomposition.
The scientists began by synthesizing a cyclocarbon base. They first protected the reactive triple bonds in the starting materials by coordinating them to cobalt. Next, from the resulting alkynes, the scientists assembled a catenane in which the cyclocarbon base with protected triple bonds was surrounded by three organic rings.
The scientists then set about removing the cobalt protection from the triple bonds. To do this, they tested numerous reaction conditions, eventually settling on a mixture of meta-chloroperbenzoic acid and bipyridine. They mixed this with catenane in dichloromethane, and purified the resulting product using gel permeation chromatography. Mass spectra of the resulting chromatographic solution confirmed the formation of a catenane with a free 48-membered carbon ring at its core. Carbon and hydrogen NMR spectroscopy also confirmed this.
The cyclocarbon protected by additional rings proved to be relatively stable in dilute solution—at room temperature, its half-life was 92 hours. But when chemists attempted to obtain free cyclocarbon without the additional rings and catenane structure using the same method, decomposition proceeded very rapidly—and the scientists were unable to obtain reliable evidence of its formation.
Thus, chemists obtained a 48-atom cyclocarbon entangled in a catenane in solution for the first time. The scientists note that the stabilization method they used could be useful for other unstable substances in the future.
We recently reported on how chemists used a scanning tunneling microscope to manipulate atoms to produce an 18-membered cyclocarbon.